Enyou ZhaoEnyou Zhao
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
More by Enyou Zhao, Yixuan Gu, Shaohua Fang, Li Yang, and Shin-ichi Hirano
Cite this: ACS Appl. Energy Mater. 2021, 4, 3, 2419–2429
Publication Date:February 24, 2021
Abstract
Fluoroethylene carbonate (FEC) is known as an effective additive to improve the performance of silicon-based anodes, but the improvement is rather limited in full-cell configuration. In this work, we utilize FEC as a main solvent to establish binary or ternary electrolytes and systematically investigate their electrochemical performance in LiNi0.5Co0.2Mn0.3O2∥Si/graphite full-cells. Compared with a commercial electrolyte, the cycle stability and reversibility of the full-cells are remarkably improved during the prolonged cycle test (500 cycles) at ambient temperature. The cells with FEC-based electrolytes demonstrate higher specific capacities at 55 °C for the 200 cycles galvanostatic cycle test, and it shows good rate capacity retention under high current (up to 5 C) at ambient temperature. In particular, the low-temperature performances of the full-cells are prominent; among the tested samples, the FEC/DMC (5:5) electrolyte delivers an outstanding capacity as high as 92.3 mA h g–1 at −40 °C. These results would endow such FEC-based electrolytes with great potential for practical applications.
1. Introduction
ARTICLE SECTIONS
Rechargeable lithium-ion batteries (LIBs) have been broadly investigated and commercialized as one of the most vital battery devices because of their advantages of high energy density, excellent long-cycle performance, no memory effect, and low self-discharge property. (1−3) As the demand for electric vehicles, aerospace, and portable electronics markets becomes more stringent, the requirements of LIBs in terms of safety, energy density, and environmental protection are also getting stricter. Among all the obstructions that hinder the development of LIBs, the deficiency of energy density is a crucial factor until now. (4−6) Consequently, plenty of research studies on new-generation cathode and anode materials have been carried out to promote the energy density of LIBs. (7,8)
Over the past few decades, conventional graphite anodes with low theoretical capacities (372 mA h g–1) significantly obstruct the improvement of battery energy density. (9) In all substitutes, silicon (Si) is regarded as the most promising candidate owing to the high theoretical capacity (3579 mA h g–1 for Li15Si4), low electrochemical potential (0.2–0.4 V vs Li/Li+), natural abundance, and environmentally benign property. (10,11) However, there are still several hinders restricting the employment of Si-based anodes. A primary defect is the huge volume change upon full lithiation of Si (large than 300%), and the violent expansion/contraction progress during lithiation/delithiation not only leads to severe particle cracking but also the pulverization of anode material, finally causing the loss of electrical contacts. (13) Meanwhile, even blend with a small amount of silicon could bring on the destruction of the anode, and the exposed fresh material constantly consumes electrolyte to make up the broken solid electrolyte interface (SEI) film, which accelerates the battery failure. (12,13) Moreover, the inherent low conductivity (10–5 to 10–3 S cm–1) and low lithium-ion (Li+) diffusion coefficient (10–14 to 10–13 cm2 s–1) of silicon material also affect the rate performance and Coulombic efficiency (CE) of batteries. (14) In response to the challenges mentioned above, approaches have been extensively explored in the past few decades.
Researchers have adopted multifarious effective strategies on anode active materials including employment of nanoscale Si particles and thin films, structural designing with carbon such as core–shell and yolk–shell structures, and composition with other alloy materials. (15−18) As another vital component, binders can restrain the expansion and crack of Si in the anode. Except for poly(acrylic acid) and sodium carboxymethyl cellulose, some biopolymer binders including alginate-based, chitosan-based, and B-cyclodextrin polymer-based binders also have been demonstrated to be effective in enhancing the cycle stability of the cells with Si-based anodes. (19,20) Furthermore, the designing of the electrolyte is of great significance for high efficiency as an economical solution. A trace amount of additive can facilitate the formation of robust SEI, which is essential to the cycling stability of batteries. (20,21) Based on excellent film-forming ability, electrolyte additives for Si-based anodes can be divided into the following categories: carbonate additives including vinylene carbonate and fluoroethylene carbonate (FEC), (22−25) lithium salt additives like lithium bis(oxalato)borate and lithium difluoro(oxalato)borate, (26,27) silanes additives such as vinyl tris(2-methoxyethoxy)silane and trimethoxy methyl silane, (28,29) Lewis acid additive [like tris(pentafluorophenyl)borate], and anhydride additives (like succinic anhydride). (30,31)
Among all the additives, FEC has been widely adopted to enhance the cycle stability of batteries with Si-based anodes, but FEC is consumed continuously and a sudden capacity drop is usually shown in long cycling tests. (23,32) Interestingly, FEC can be configurated as the main solvent (>20 wt %) in electrolytes own to the high dielectric constant and high oxidation potential, meanwhile, the great electrode compatibility with both graphite and Si anodes. (33−40) Some research studies have demonstrated that FEC used as a main solvent can effectively improve the battery cycle performances with Si-based anodes. However, the majority of the contributions reported so far are based on half-cell configuration separated from the influence of cathodes, which is not equivalent to the battery performances in practical applications. (33−35,41,42) Although several measurements are adopted by harsh full-cell operations, the evaluation of batteries is restricted in cycle tests with limited cycle numbers (not exceed 150 cycles), far below the requirements of practical requirement. (36−38,40) The specific linear carbonates are always chosen to evaluate the influence of FEC, but the impact of species and proportions of different linear carbonates on electrochemical performances have not been investigated. Also, commercial electrolytes based on ethylene carbonate (EC) shows poor capacity below −20 °C and severe capacity decay higher than 50 °C as the result of the high melting point of EC (Tm = 38 °C) and higher kinetics at elevated temperatures. (31,62,63) When substituting EC with FEC, it is promising to establish advanced electrolytes that function well with full-cells with Si-based anodes in a wider temperature range satisfying practical conditions.
Herein, 15 binary and 3 ternary FEC-based electrolytes are formulated by FEC (in gradient contents), linear carbonates, and LiPF6. After the confirmation of the property conductivity and good wettability of the electrolytes, LiNi0.5Co0.2Mn0.3O2∥Si/Graphite cells are assembled to make a further systematic electrochemical evaluation. Compared with a commercial electrolyte, the prolonged cycle tests at both ambient temperature (500 cycles at 25 °C) and elevated temperature (200 cycles at 55 °C) show better cycle performances with FEC-based electrolytes. Under high current density (up to 5 C), cells with FEC as main solvent display similar rate capacity retentions compared with EC/DMC electrolyte. Particularly, the low-temperature performance of the cells with FEC-based electrolytes is remarkable and could maintain high discharge capacities at even −40 °C.
2. Experimental Section
ARTICLE SECTIONS
2.1. Materials
Dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), diethyl carbonate (DEC), and LiPF6 were purchased from Zhangjiagang Guotai-Huarong Co., Ltd. FEC was provided by Shanghai Titan Technology Co., Ltd. 1 M LiPF6 was dissolved in the mixture of FEC, DMC, EMC, and DEC to obtain FEC-based binary and ternary electrolytes for LIBs (in the following, the concentration of LiPF6 is 1 M in all electrolytes). As a reference, the commercial electrolyte with 1 M LiPF6 in EC/DMC (50:50 wt %) was purchased from Zhangjiagang Guotai-Huarong Co., Ltd. Conservation of solvents and formation of electrolytes were all implemented in a glovebox filled with argon (H2O < 0.1 ppm, O2 < 0.1 ppm). Cathode-active material LiNi0.5Co0.2Mn0.3O2 (NCM523) was purchased from BASF, and silicon/graphite (Si/Gr) used as the anode material was purchased from BTR New Energy Materials Inc., China.
2.2. Measurements of Electrolyte Physical Properties
The conductivity, viscosity, and wettability of electrolytes were tested at 25 °C. The conductivity was obtained by a DDS-11A conductivity meter, and the viscosity was measured by a DV-III ULTRA viscometer (Brookfield Engineering Laboratories, Inc). The wettability of the electrolyte to the separator and electrodes was measured by a contact angle measuring instrument (OCA20, Data Physics). A differential scanning calorimetry (DSC, TA Instrument Q2000) was conducted to confirm the freezing point of electrolytes, and about 10 mg of the electrolyte was cooled from 20 to −80 °C at the rate of 1 °C min–1 after sealing in a small aluminum crucible. The thermal stability of electrolytes and electrodes was measured on a Photo-DSC 204F1 (NETZSCH, Germany) at the heating rate of 5 °C min–1. Full-cells were fully charged to 4.2 V after two cycles and dissembled in the glovebox. The cathodes and anodes were rinsed three times after being soaked in pure DMC for 10 h to remove the residual electrolyte and dried under vacuum. Then, the electrode materials were scraped from the current collectors and the resulting electrodes (∼2.0 mg anode material and ∼4.0 mg cathode material) were sealed together with corresponding electrolytes (∼8.0 mg) in a high pressure sealed pan.
2.3. Battery Assembly and Electrochemical Measurements
CR-2016 coin cells were assembled to evaluate the electrochemical performance of electrolytes in NCM523∥Si/Gr cells on a Land CT2001A cell test instrument. The cathode consistent of 95.0 wt % LiNi0.5Co0.2Mn0.3O2 active materials, 3.0 wt % polyvinylidene fluoride binder, and 2.0 wt % carbon black were deposited onto the aluminum foil with a loading of 9.4 mg cm–2 (1.38 mA h cm–2). The anode composed of 3.0 wt % carboxy methyl cellulose and 3.0 wt % styrene butadiene rubber as binders, 8.0 wt % Super P as the conducting agent, and 86.0 wt % silicon/graphite as the active material was coated on copper foils with a loading of 4.4 mg cm–2 (1.59 mA h cm–2). The capacity ratio between the Si/Gr anode and NCM523 cathode (N/P ratio) is 1.15. The coin cells were assembled in the glovebox with a polyethylene separator, and the charge–discharge (C–D) tests were measured between 2.5 and 4.2 V at a constant current. The current rate was calculated according to the loading mass of the active cathode material and the nominal capacity of NCM523 (155 mA h g–1). The cells were aged for 16 h at room temperature before the test to ensure the complete penetration of the electrolytes, and a three-cycle activation was performed at 0.1 C.
The electrochemical impedance spectroscopy (EIS) test was conducted on a CHI660D electrochemistry workstation, and the full-cells are tested at the open circuit potential after being cycled for three times at 0.1 C. The components of SEI films on the Si–Gr anode surface after activation were further investigated by X-ray photoelectron spectroscopy (XPS) tests on an AXIS Ultra DLD (Kratos, England) with aluminum K X-ray radiation. Scanning electron microscopy (SEM) images of the anode and cathode surface were taken by a scanning electron microscope (Nova NanoSEM NPE218) with an energy-dispersive spectrometer (EDS) before and after C–D tests. The dissolution of transition metals on the anode was measured by ICP-OES (Thermo Scientific iCAP 7600). Before the SEM, XPS, and ICP test, the electrodes were immersed in DMC solvent for 10 h, then rinsed with DMC solvent more than three times to eliminate the remaining LiPF6 and residual electrolytes, and finally dried under vacuum.
For the reassembling test, the full-cells were dissembled after 300 cycles at 1 C and cycled for another three cycles at 0.1 C before being disassembled to evaluate the accurate specific capacity. Then, the cycled anode and cathode are matched with lithium plates to make half-cells separately, and the same electrolytes as used in the long cycle test were adopted to measure their lithium-ion storage ability. To compare with pristine electrodes, the discharge capacity of reassembled half-cells with both cycled cathodes and anodes is measured at 0.05 C.
3. Results and Discussion
ARTICLE SECTIONS
At first, the conductivities of 16 binary and 3 ternary electrolytes are measured at 25 °C, and the results of FEC-based binary samples are displayed in Figure 1. For example, the conductivities of FEC/DMC, FEC/EMC, and FEC/DEC at the weight ratio of 5:5 are 10.00, 8.35, and 7.14 mS cm–1, respectively. In general, the conductivity reflects the dissociation ability of Li+ in electrolytes, and high dielectric constant as well as low viscosity commonly beneficial to the high conductivities in carbonate solvent electrolytes. (43−45) At 25 °C, the dielectric constant of DMC, EMC, DEC continuously decrease in order, and the viscosities increase sequentially. (52) It can be explained that the conductivities of all electrolytes decrease as the chain length of linear carbonates increases with the same FEC content. Besides, all binary FEC-based electrolytes possess the highest conductivities when the content of FEC reaches 40 wt %, which is the same as EC-based electrolytes. (44) The viscosity of electrolytes rises with the content of FEC continuously increasing, as listed in Table S1, and the compromise of both dielectric constant and viscosity reflects on the highest conductivity at a specific proportion. (43) Among 15 FEC-based binary electrolytes, the FEC/DMC (4:6) sample has the highest conductivity of 10.34 mS cm–1, which is comparable to the value of commercial electrolyte. For ternary electrolytes, their conductivities are close, and the highest value is 9.22 mS cm–1 occurring in the FEC/DMC/EMC (1:1:1) electrolyte (specific statistics are listed in Table S2).

For LIBs, the good wettability of the electrolyte is conductive to improve its affinity with separators and electrodes, thereby promoting the transportation of Li+ and making the best use of active material. The contact angles of 10 binary and 3 ternary samples on the separator are measured, and the contact angles of different electrolytes all increase with the rise of the FEC content, as listed in Table S3. For example, when the content of FEC is 50 wt %, as illustrated in Figure 2b–d, the contact angles of EC/DMC, FEC/DMC, FEC/EMC, and FEC/DEC electrolytes are 44.3, 42.1, 37.1, and 41.1°, respectively. In the main, lower surface tension leads to better wettability and reflects in lower contact angles. (46,47) Compared with EC (50.6 mN m–1 at 40 °C), (48,49) the lower surface tension of FEC (46.1 mN m–1) leads to the lower contact angles of FEC-based electrolytes. The surface tensions of DMC, EMC, and DEC are 28.5, 25.9, 26.3 mN m–1, respectively. (50−52) For all of the binary electrolytes, the contact angle increases with the rise of FEC content for its high surface tension. For ternary electrolytes, the FEC/EMC/DEC (1:1:1) sample shows the best wettability with the lowest contact angle of 36.5°. The dynamic penetration processes into electrodes of EC/DMC (5:5) and FEC/DMC (5:5) electrolytes are further recorded, and better wettability is beneficial to the penetration. As shown in Figure 2e–h, the penetrations of FEC/DMC into the NCM523 cathode is faster than the EC/DMC electrolyte, and similar results can be observed on the Si/Gr anode. In conclusion, employing FEC instead of EC as the main solvent with linear carbonates can improve the wettability of electrolytes for both separators and electrodes.

To further evaluate the electrochemical performances of FEC-based electrolytes, the prolonged cycle test of NCM523∥Si/Gr cells is carried out. An initial activation process is conducted at 0.1 C for three cycles, and the initial charge–discharge (C–D) curves of binary electrolytes with 50 wt % FEC are displayed in Figure S1. The curves of FEC/EMC and FEC/DEC samples overlapped with each other, and a voltage platform appears at 3.52 V during the charging process, which is a little higher than FEC/DMC and EC/DMC samples. It reflects that the ethyl ester group of EMC and DEC causes the higher charging voltage platform compared with DMC, and the polarization of batteries containing high-conductivity solvent FEC/DMC is relatively better. The initial Coulombic efficiencies (ICEs) of all samples are calculated and displayed in Figure S2, and FEC-based electrolytes have higher ICEs at around 86.0% compared with EC/DMC (84.8%). It indicates that the consumption of FEC-based electrolytes is comparably lower in the first C-D cycle and beneficial to the full utilization of lithium ions.
The components of the SEI film on the Si–Gr anode after three cycles at 0.1 C are determined by XPS measurements, as shown in Figure S3. For the EC/DMC (5:5) electrolyte, four peaks can be observed in C 1s spectrum at 284.8, 286.2, 288.2, and 289.7 eV, which are related to C–H bonds in hydrocarbons, C–C–O bonds in carbonates or ethers, R–CO3 bonds in alkyl carbonates, and the bond of Li2CO3, respectively. (37,53) In the O 1s spectrum, two peaks are relevant to C═O (531.5 eV) and C–O bonds (532.3 eV). (61) Three peaks in the F 1s spectrum are related to Li–F bonds(685.0 eV), P–F–O bonds(686.5 eV), and C–F bonds(689.0 eV). (54,55) In general, there is no obvious difference in the types of peaks in spectra between EC and FEC-based samples except the content of Li–F, which is significantly higher in FEC-based samples. LiF-rich SEI is regarded as facilitating to suppress the volume change partially during cycling, hence improving the cycle performance. (54,56) The result shows that the surfaces of anodes with different electrolytes are similar in types of organic compounds; however, the content of the chemical bonds is different in SEI films especially for Li–F, which may affect the long cycling performance of the battery.
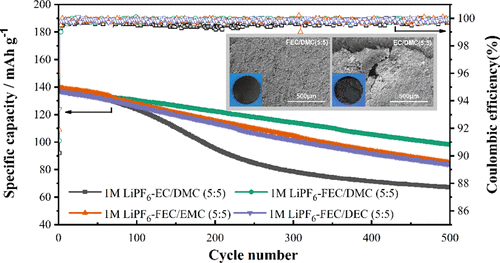
The 500 cycles C–D test of NCM523∥Si/Gr cells at 1 C is performed at ambient temperature (25 °C), and the results are listed in Table S4. For a comprehensive comparison, three different linear carbonates at five concentration gradients are chosen to match with FEC for formulating binary electrolytes. The specific capacities and CEs of binary electrolytes with 50 wt % FEC are displayed in Figure 3a, and it can be observed that the reversible capacities of FEC-based samples are all higher than the EC/DMC electrolyte (67.1 mA h g–1) after 500 cycles—among them, the FEC/DMC sample has the highest specific capacity of 96.1 mA h g–1. The pattern of the battery capacity fading process with EC/DMC electrolyte is significantly different from FEC-based samples in the prolonged cycle test. For the cell with EC/DMC electrolyte, a severe capacity decay appears between 50th and 300th cycle, and the CE of the cells drops down then stays around 99.5% during this period. However, the CE of FEC-based samples with different linear carbonate reaches and stays higher than 99.8% after the initial 10 cycles, thus maintaining good cyclic stability in the whole cycle test. Among FEC-based samples, even the initial specific capacity of the FEC/EMC sample is higher, the capacity decay of the battery with the FEC/DMC electrolyte is relatively slower in the whole progress. In consequence, the capacity retentions of the cells with FEC-based electrolytes are much higher than the EC/DMC (48.1%) electrolyte after 500 cycles, and for the FEC/DMC (5:5) sample, the value can reach up to 71.4%.

As a main solvent in electrolytes, the content of FEC is another vital factor for battery performance, and the FEC/DMC series are selected for further discussion, as presented in Figure 3b. Although the cells with low FEC content have higher specific capacity at the beginning for the high conductivity, the capacity decay of the FEC/DMC (5:5) sample is relatively slow as cycling and has the maximum specific capacity after 500 cycles. To further investigate the influence of electrolyte components on the battery cycle performance, the capacity retentions after 500 cycles of all samples are calculated, as shown in Figure 3c. The capacity retention of the cells rises up and then drops down with FEC content increasing in all FEC-based binary electrolyte series, but the optimal content ratio of electrolytes with various linear carbonates is different. At the optimal ratios, the best capacity retentions of FEC/DMC (5:5), FEC/EMC (6:4), and FEC/DEC (7:3) samples are 71.4, 67.3, and 65.8%, respectively. In short, the FEC/DMC electrolyte system is excellent in the long cycle performance of NCM523∥Si/Gr full-cells (specific statistics are listed in Table S5).
Although the cycle stability of the cells has been greatly improved by FEC-based electrolytes, the reversible capacity continues to decline during the cycle test. The SEM and digital photographs are analyzed for the surface structure of electrodes before and after the 500 cycles C–D test, as shown in Figures 4 and S6. The cross-section picture of the pristine anode is displayed in Figure 4a by combining the photographs of SEM and EDS, and it is observed that the silicon material is dispersed in the gap between flake graphite. As can be seen from Figure 4b–f, the smooth and clear surface of the pristine state Si–Gr anode is confirmed before cycling, but the surface of graphite is covered with a thick layer of electrolyte decomposition in all samples after 500 cycles, and the irregular vesicles are the cycled silicon particles. Compared with the EC/DMC electrolyte, the graphite anode surface of the FEC-based electrolytes is denser and with many granular sediments rather than powders. As demonstrated in digital photographs, the surface of the cycled anode with EC/DMC electrolyte is unconsolidated even partly peeled off, and the damaged area could account for one-third of the anode surface. However, the intact and flat surface can be identified clearly in the samples with main-solvent FEC regardless of linear carbonate species, as shown in Figure S6. It is confirmed that FEC-based electrolytes can protect the anode structure efficiently. (36,37,42) The discrepancy also means that the drastic volume change of anode with EC/DMC electrolyte not only affects the activity of silicon but also harmful to the whole anode structure. Furthermore, FEC is conducive to the film-forming process for its high reduction potential, and the decreased lowest unoccupied molecular orbitals energy means that it can decompose before EC to makes a dense and elastic SEI film as reported. (38,57,58) In summary, FEC could facilitate to form of a robust SEI film on the anode that can reduce the side reaction as well as suppress the volume change thus protect the anode structure, while the SEI film with the EC-based electrolyte is not strong enough to buffer the drastic volume changes and finally result in the severe structure destroy.

Besides the anodes, the SEM characterization of NCM523 cathodes is also conducted before and after cycling, as illustrated in Figure S7. The big LiNi0.5Co0.2Mn0.3O2 assembled spheres are surrounded by small particles of carbon black, and binder can be observed on the cathode in the pristine state. After the prolonged C–D test, there is no obvious difference on the surface of cathodes between electrolytes, which reveals that the cathodes maintain the structural integrity in all samples. The dissolution of transition metal is known as a major cause for the capacity decay in the battery system with the NCM cathode, and the clear surface may not be enough to reflect the situation of the cathode. Therefore, the ICP-OES test is conducted to measure the sediment amount of three transmission metals (Ni, Co, and Mn) on the cycled Si/Gr anode. As summarized in Figure 5, the amount of Co is decreased compared with the reference group, although there are more Ni and Mn ions detected in the FEC-based sample, which is similar to the high-temperature storage test in the FEC/DEC system. (53) Co is commonly regarded as the crucial metal to keep the stable structure of the NCM cathode, thus we speculate that the dissolution of Co is inhibited in the FEC-based electrolyte, but the other two transition-metal ions are more dissolved.

The reassembling tests of the cycled cells with FEC/DMC (5:5) and EC/DMC (5:5) electrolytes are put forward to find out the main culprit for the capacity decay. Since the severe structural damage of the anode makes it hard to be completely peeled off from the separator after 500 cycles, in the meantime, a large difference has emerged in reversible capacity between EC/DMC and FEC-based samples, the cells are disassembled in the glovebox after 300 cycles at 1 C. As displayed in Table 1, the specific capacity of full-cell after 300 cycles with EC/DMC electrolyte has dropped to 80.3 mA h g–1, and the FEC/DMC electrolyte is 109.0 mA h g–1 with relatively high capacity retention of 77.6%. As for the cathode, the specific capacity of the half-cell in the pristine state is 151.4 mA h g–1 and reassembled half-cells with EC/DMC and FEC/DMC electrolytes show specific capacities of 150.1 and 150.6 mA h g–1, respectively. Meanwhile, the charge capacities of cycled cathodes of reassembled half-cells are similar to the discharge capacities of full-cells before being disassembled, which indicates that the Li+ storage ability of cathodes remains well after the cycle test with both electrolytes. However, the reversible Li+ storage in cathodes has dramatically reduced from the charge capacity of the half-cells with cycled cathodes, which may be caused by the parasitic reaction of electrolytes or the destroying of the anode. To measure the residual capacity of anodes, the cycled anode attached with the separator was peeled off carefully and then reassembled with a lithium plate. The specific capacity of the pristine anode is 425.8 mA h g–1 at 0.05 C, and the specific capacity of the reassembled half-cell with the EC/DMC electrolyte and FEC/DMC electrolyte is 357.6 and 395.7 mA h g–1, respectively. The irreversible capacity loss of the anodes is non-negligible, and the damage of the cycled anode with EC/DMC is obviously more serious than the FEC/DMC electrolyte. The retentiona and retentionb in Table 1 show the Li+ storage ability of cathode and anode after 300 cycles, respectively, and both of them are much higher than the capacity retentions of full-cells before being disassembled. It indicates that the capacity decay of full-cells is not only originated from the structural collapse of electrodes but also affected by continuous consumption of Li+ of side reactions. (59,60) Since the cathode can maintain the high reversible capacity in reassembled half-cells, the difference between the capacity retention of full-cells and the retention of half-cells with cycled anodes can reflect the consumption of Li+ during cycling. With the main-solvent FEC, this difference (92.9% – 77.6% = 15.3%) is distinct with EC/DMC sample (84.0% – 57.7% = 26.3%), which reveals that the FEC-based electrolyte can not only protect the anodes structure but also inhibit the consumption of Li+. In conclusion, the Li+ storage capacity of the cathode remains well during the prolonged cycling test for both two samples, and FEC-based electrolytes can effectively protect the anode structure as well as reduce the continuous consumption of Li+, ultimately improving the cycle life of the full-cells.
Table 1. Battery Performances of Full-Cells After 300 Cycles, and the Statistics of Reassembled Half-Cells with Cycled Cathodes and Anodes
| full-cell | anode (half-cell) | cathode (half-cell) | |||||
|---|---|---|---|---|---|---|---|
| sample | discharge capacity (mA h g–1) | capacity retention (%) | discharge capacity (mA h g–1) | retentiona (%) | charge capacity (mA h g–1) | discharge capacity (mA h g–1) | retentionb (%) |
| pristine | 425.8 | 151.4 | |||||
| EC/DMC (5:5) | 80.3 | 57.7 | 357.6 | 84.0 | 82.5 | 150.1 | 99.1 |
| FEC/DMC (5:5) | 109.0 | 77.6 | 395.7 | 92.9 | 112.3 | 150.6 | 99.5 |
a
Retentiona is calculated by the discharge capacity of reassembled half-cells with cycled anode divide by the discharge capacity of the pristine anode (e.g., 357.6/425.8 × 100% = 84.0%).
b
Retentionb is calculated by the discharge capacity of reassembled half-cell with cycled cathode divided by the discharge capacity of pristine cathode.
After three cycles at 0.1 C for activation, the specific capacities of NCM523∥Si/Gr cells with 10 binary and 3 ternary electrolytes are obtained by the continuous C–D test from 1 to 5 C at ambient temperature. The specific capacities of the batteries with binary electrolytes containing 50 wt % FEC are shown in Figure 6a, and it is found that the rate performance of batteries with FEC/DMC and FEC/EMC electrolytes is similar to the reference group and the FEC/DEC sample has lower specific capacity under high current. To systematically evaluate the rate performance of various electrolytes, rate capacity retentions of 5 C/0.1 C are calculated, as illustrated in Figure 6c, and statistics are listed in Table S6. When the mass ratio is 5:5, rate capacity retentions of the cells with EC/DMC, FEC/DMC, FEC/EMC, and FEC/DEC electrolytes are 78.73, 79.19, 78.70, and 72.47%, respectively. It shows that FEC-based electrolytes have great rate performance as similar as the EC/DMC sample up to 5 C. The rate performance of batteries is commonly affected by both conductivity of electrolyte and interface impedance, thus EIS tests are implemented at an open circuit of NCM523∥Si/Gr cells after cycling three times at 0.1 C. As demonstrated in Figure 6b, the EIS is fitted according to the equivalent circuit, (61) in which the Rb, RSEI, and Rct are the bulk resistance of the electrolyte, the resistance of the SEI film, and the charge-transfer resistance, respectively. Table 2 lists the statistic of RSEI and total interfacial resistance (Ri = RSEI + Rct). Since there is a large difference between the electrolyte conductivities as mentioned above, the big disparities of rate performances between the samples with DMC and DEC are mainly accounted for the conductivities. In the meantime, the substantial reduction of interfacial resistance and proper conductivity making the FEC/EMC sample also has a relatively higher rate performance at 5 C. The Ri of the samples all rise when the content of FEC drops to 40 and 30 wt %, which reflects on the poor rate capacity retentions in full-cells even with relatively high conductivity. As for ternary electrolytes, the rate capacity retentions of the cells are slightly better than binary samples and close to each other. The high conductivities and low interface resistances of FEC/DMC/EMC and FEC/DMC/DEC electrolytes account for their relatively higher rate capacity retentions.

Table 2. Resistance Parameters of Binary Electrolytes at Ratio 5:5 Calculated from the EIS Plots
| electrolyte | Rb/Ω | RSEI/Ω | Rct/Ω | Ri/Ω |
|---|---|---|---|---|
| 1 M LiPF6-EC/DMC (5:5) | 3.23 | 0.77 | 1.39 | 2.16 |
| 1 M LiPF6-FEC/DMC (5:5) | 3.29 | 0.79 | 1.90 | 2.69 |
| 1 M LiPF6-FEC/EMC (5:5) | 3.47 | 0.51 | 0.56 | 1.07 |
| 1 M LiPF6-FEC/DEC (5:5) | 2.72 | 0.55 | 0.96 | 1.51 |
In comparison, the FEC-based electrolytes with the same linear carbonate content (50 wt %) as the reference group are selected to further investigate the performances of batteries at elevated and low temperatures. The 200 cycles C–D test of NCM523∥Si/Gr cells at 1 C is performed at 55 °C, as displayed in Figure 7. Compared with EC/DMC, the cells with FEC-based electrolytes exhibit the obvious dominance in the initial stages, and the capacity retentions are impressively improved after 50 and 100 cycles (Table S8) as reported. (62,63) However, the discrepancy of specific capacities between the full-cells with FEC- and EC-based electrolytes gradually diminishes when the test prolongs to 200 cycles. Among them, the FEC/DMC sample has the highest irreversible capacity of 96.4 mA h g–1 (with the capacity retentions of 64.2%). It is obvious that the speed of capacity decay of all samples is apparently accelerated at elevated temperatures, and the comparatively lower CE (∼99.6%) verifies the result. On the one hand, the diffusion speed of electrolytes in the anode is accelerated with the enhancement of temperature, and it may cause the fast growth of SEI films to accompany consuming Li+ especially on the anode with silicon. On the other hand, the hydrolysis of LiPF6 and the dissolution of the cathode material in the electrolyte are aggravated at high temperatures and further participate in side reactions on the anode. (55,62,63) Several researchers found that the FEC defluorination makes the increase of HF at elevated temperatures, which causes severe dissolution of transition-metal ion and result in constant capacity decay. (60−64) Thus, we suspect that the dominant of FEC-based electrolyte at high temperature is because of a comparatively robust SEI which could inhibit the consumption of the electrolyte. After 200 cycles, SEM images and digital photographs are collected to investigate the structural changes on the surface of anodes (Figure S9). It is easy to find that the surface of the cycled anode with the EC/DMC electrolyte is loose and rough, and many bumps can be observed in the digital photograph, while the surfaces of all FEC-based samples are clean and smooth. From the low-magnification SEM images, a dense SEI film on the anode can be observed with FEC-based electrolytes. (58) Through the high-magnification inset graph in SEM images, many bubble-like spherical particles appear on the surface of the anode with EC/DMC, while the intact anode surface is covered with spot-like deposits after cycling with FEC-based electrolytes. In short, although the severe side reactions accelerate the capacity decay, FEC used as the main solvent can stabilize the electrochemical behavior of the battery and improve capacity retention at elevated temperatures for 200 cycles.

The thermal stability of fully charged electrodes and electrolytes is of great significance to battery safety, and the DSC measurement is further carried out. Figure S10a shows the DSC curves of EC/DMC (5:5) and FEC/DMC (5:5) electrolytes. It can be seen that the main decomposition peak of both electrolytes exceeds 200 °C, which resulted from the decomposition of LiPF6 and the side reactions with cyclic carbonate. (65) Due to the decomposition of LiPF6 and FEC and the side reactions between the decomposition products, the exothermic peak of the FEC-based electrolyte appears at a lower temperature. (55,66) The thermal stability of the lithiated anode and delithiated cathode materials with or without electrolyte is displayed in Figure S10b,c. Although there is no obvious exothermic peak observed in the curves of the two samples without electrolyte, the peaks of the fully charged electrodes immersed in corresponding electrolytes are present within the set temperature range. The first exothermic peaks of both two samples appear at 80 to 120 °C, which is caused by the reactions of HF produced by the decomposition of LiPF6 with the component of SEI films. (66,67) Interestingly, the difference of initial decomposition temperature between the two samples is significantly reduced compared to pure electrolytes, and it is observed that the peak area of the FEC/DMC (5:5) sample is smaller, which could be explained by its comparatively stable SEI film. However, with the presence of the fully charged electrode materials, the decomposition of LiPF6 in FEC-based electrolyte releases HF and leads to its lower initial exothermic temperature. (68,69) The exothermic peaks between 250 and 350 °C are attributed to the oxidation reaction between the electrolyte and fully lithiated Si–Gr anode as well as delithiated NCM cathode. (66,68) In short, the thermal stability of FEC/DMC (5:5) is not as good as the EC/DMC (5:5) samples.
Figure 8a demonstrates the conductivities of EC/DMC and FEC-based electrolytes from 50 to −70 °C. The conductivities of all electrolytes drop slowly with the decrease of temperature in the beginning phase, but the curve of the EC/DMC sample declines sharply at around −40 °C due to the freezing of the electrolyte, as shown in Figure S11a. As for FEC-based electrolyte, except for a crystalline peak of FEC/DMC electrolyte appear lower than −60 °C, no freezing point is observed within the set temperature range in the DSC test. It means that FEC-based electrolytes maintain the liquid phase before the temperature drops to −60 °C. Consequently, FEC-based electrolytes have much higher conductivities compared with EC/DMC at low temperature, and the FEC/DMC electrolyte has the highest conductivity in the large temperature range from 10 to −50 °C.

The low-temperature performance of the NCM523∥Si/Gr cells with binary electrolytes is measured at 0.1 C from 25 to −40 °C. As the temperature decreases, the discharge curves of the cells with EC/DMC and FEC/DMC electrolytes are observed and illustrated in Figure 8c,d, and the curves of FEC/EMC and FEC/DEC electrolytes are shown in Figure S11b,c. The operating voltage continues to decline, and the discharge capacity gradually reduces along with temperature drop. It is because the resistance of the cell increases at low temperatures, the polarization becomes more serious and the electrochemical reactions get slow down, eventually leading to the decline of the operating voltage. (33,63) As known, the SEI on the anode of the FEC-based electrolyte contains more inorganic contents such as LiF, which could enhance the conductive properties and leads to better low-temperature performance. (66) Therefore, the capacity of the cell with the EC/DMC electrolyte sharply falls to 40 mA h g–1 at −30 °C, and the cells almost cannot work as the temperature continues to drop. Comparatively, the cells with FEC-based electrolytes show significant low-temperature electrochemical performance. The specific capacities of all FEC-based samples remain higher than 70.0 mA h g–1 even it comes to −40 °C, and the FEC/DMC sample is the highest one of 92.3 mA h g–1. Temperature dependency of discharge capacity is further calculated, as displayed in Figure 8b, and it is obvious that the normalized capacities of FEC-based electrolytes have a great advantage in low-temperature performance. Since the low freezing point, high conductivity, and better interfacial properties, the cells with FEC-based electrolytes reveal excellent low-temperature performance outperformed that of the EC/DMC electrolyte.
4. Conclusions
ARTICLE SECTIONS
In summary, a series of FEC-based binary and ternary electrolytes are formulated to systematically evaluate the electrochemical performances of LiNi0.5Co0.2Mn0.3O2∥Si/Graphite cells. At ambient temperature, FEC-based electrolytes can dramatically stabilize the cycling behavior of full-cells in 500 cycles compared with a commercial electrolyte. The reassembling test of cycled anodes and cathodes proves that electrolytes with main-solvent FEC can significantly restrain the destruction of Si/Gr anodes as well as irreversible consumption of lithium ions, which further inhibit the severe capacity decay. Moreover, the full-cells with FEC-based electrolytes exhibit better cycle performances at an elevated temperature (55 °C) as well as good rate performance. In particular, the low-temperature performance of the cells with main-solvent FEC is remarkably improved and maintains high discharge capacities even at −40 °C.
【Article link】
https://doi.org/10.1021/acsaem.0c02946