Yue ZouYue Zou
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
More by Yue Zou, Gaopan Liu, Ke Zhou, Jing Zhang, Tianpeng Jiao, Xiaozhen Zhang, Yong Yang, and Jianming Zheng*
Cite this: ACS Appl. Energy Mater. 2021, 4, 10, 11051–11061
Publication Date:September 22, 2021
Abstract
Ni-rich LiNixCoyMnzO2 (NCM, xNi ≥ 0.9) layered materials are appealing cathode candidates for future-generation higher-energy-density lithium-ion batteries (LIBs). However, structural and interfacial instability of Ni-rich cathodes needs to be addressed prior to the large-scale deployment in electric vehicles. Herein, a diboron electrolyte additive 5,5,5′,5′-tetramethyl-2,2′-bi-1,3,2-dioxaborinane (TBDB) is proposed for constructing a stable LiNi0.9Co0.05Mn0.05O2 (NCM90)/electrolyte interface, thus improving the long-term cycle life and rate capability of an NCM90 cathode. Using an optimized 0.3% TBDB-containing electrolyte, the NCM90 cathode shows a capacity retention up to 84.3% after 200 cycles at 1C at a charge cutoff of 4.4 V, much superior over 70.1% when using the baseline electrolyte. The obvious performance improvement could be explained by the fact that TBDB with higher highest occupied molecular orbital (HOMO) energy is preferentially oxidized before electrolyte solvents, through boron–boron bond cleavage, generating a compact, thin, and protective cathode electrolyte interphase (CEI) layer on the cathode electrode. As a result, the electrolyte decomposition is effectively restrained with fewer poorly conducting byproducts being accumulated, which significantly enhances the interfacial stability and redox reaction kinetics of a Ni-rich NCM90 cathode.
1. Introduction
It has been widely recognized that to reduce the consumption of fossil fuels, replacing internal combustion engine cars by electric vehicles (EVs) has become the main trend from environmental protection and sustainable development point of view. (1−4) To realize the goal of “EV everywhere”, lithium-ion batteries (LIBs) of higher energy density are urgently demanded, to relieve the mileage anxiety. In particular, more attention has been paid to exploring alternative cathodes, which somewhat dictates the energy density boost of LIBs. (5−9) It is well established that increasing the charge cutoff voltage and/or developing Ni-rich layered cathodes LiNixCoyMnzO2 (NCM) are effective strategies to promote the energy density of LIBs. Increasing the cutoff voltage indeed can deintercalate more lithium ions out of the active cathode material and thus increases the discharge capacity. For instance, when the charge cutoff voltage is increased to 4.8 V vs Li/Li+, LiNi0.4Co0.2Mn0.4O2 (NCM424) is capable of delivering an initial discharge capacity of 206 mAh g–1, obviously higher than that of 185 mAh g–1 at 4.5 V. (10) However, this is at the expense of faster capacity decay owing to the accelerated structural/interfacial degradation. Alternatively, Ni-rich NCM layered cathodes allow more Ni3+/4+ redox reactions to occur without significant oxygen evolution, enabling higher discharge capacity and energy density. The cathode of the Ni content xNi ≥ 0.9, e.g., Li[Ni0.9Co0.045Mn0.045Al0.01]O2 (NCMA90) is able to deliver a discharge capacity of 228 mAh g–1 at 0.1C at a charge limit of 4.3 V, well exceeding LiNi0.8Co0.1Mn0.1O2 (NCM811) (206 mAh g–1). (11)
Nevertheless, several technical challenges still remain with the practical application of a Ni-rich cathode of a Ni content xNi ≥ 0.9 for being deployed in EVs. (12−14) On the one hand, Ni-rich cathode experienced particle cracking (esp. for secondary particles) and irreversible structural transformation from a layered to disordered rock-salt phase. (15−17) On the other hand, the oxidative decomposition of an electrolyte readily results in continuous accumulation of a resistive passivation film on the cathode surface, causing a rapid build-up of interfacial resistance. (18) As a result, LIBs using Ni-rich cathodes show relatively fast deterioration of electrochemical performance during cycling.
To date, several strategies have been proved useful to address the abovementioned issues, including cation doping, (19,20) surface coating, (21,22) and functional electrolytes. (23,24) Notably, designing a functional electrolyte is a crucial and scalable approach to build a stable and protective cathode and an electrolyte interface (CEI) film. (25) Among different kinds of electrolyte additives, boron-containing ones have attracted extensive attention due to their good CEI film-forming ability. In principle, they possess relatively higher occupied molecular orbitals (HOMOs) in comparison with carbonate solvents, suggesting a tendency toward preferential oxidation on the cathode. (26) Additionally, owing to the electrophilic property of boron, boron-type additives also help to stabilize the LiPF6 salt by trapping PF6– and F–, hence depressing the generation of LiF, Li2CO3, and hydrofluoric acid (HF). (27) As a representative example, addition of a 2% lithium bis(oxalato)borate (LiBOB) additive expedited the formation of a favorable CEI layer that helped to attain a high capacity retention of 96.8% after 200 cycles at a C/3 rate. (28) In addition, triethylborate (TEB) was adopted as a film-forming additive for LiNi1/3Co1/3Mn1/3O2 (NCM333) where a TEB-derived CEI effectively protected the cathode surface against excessive side reactions. (29) Nonetheless, the optimized amount of 10% TEB (added into baseline electrolyte) would be too high to be applied in mass production. Beyond that, a trimethyl borate (TMB) additive together with tetramethylene sulfone (TMS) as synergistic electrolyte components was introduced to improve the electrochemical performance of the LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode at 4.5 V. (30)
Considering the unique features of a boron-type additive, here, we propose, for the first time, a novel boron-containing additive, 5,5,5′,5′-tetramethyl-2,2′-bi-1,3,2-dioxaborinane (short as TBDB) (molecular structure is displayed in Figure 1). It is demonstrated that the TBDB additive could effectively stabilize the interface of single-crystal Ni-rich cathode Li[Ni0.9Co0.05Mn0.05]O2 (NCM90), and pronouncedly improve the electrochemical performance even with a limited amount (0.3 wt %), signifying an efficient electrolyte additive. Moreover, an in-depth understanding on the functioning mechanism of TBDB is attained, based on the careful investigation via a variety of physicochemical characterization techniques.

2. Experimental Section
2.1. Electrolyte and Electrode Preparation
Single-crystal LiNi0.9Co0.05Mn0.05O2 (NCM90) was acquired from Ronbay Technology Co., Ltd. The baseline electrolyte of 1 M LiPF6 dissolved in 30 wt % ethylene carbonate (EC)/70 wt % diethyl carbonate (DEC) was provided from Guotai Huarong Co., Ltd. Functional electrolytes containing TBDB (3A Chemical, purity: 98%) were prepared by ushering proper amounts of TBDB (0.1, 0.3, 0.5, and 1.0% by weight) into the baseline electrolyte. The ionic conductivities of the baseline electrolyte and the 0.3% TBDB-containing electrolyte are provided in Table S1. NCM90 electrodes were made of NCM90 powders, acetylene black (AB), and a poly(vinylidenefluoride) (PVDF, Aekema) binder at a mass ratio of 8:1:1, as-prepared by a slurry coating employing N-methyl-2-pyrrolidineone (NMP) as a dispersing solvent. The mixture after magnetic stirring for about 5 h was casted on an Al foil, and then the electrode was dried at 100 °C under a vacuum condition for 2 h. The areal coating weight of the NCM90 electrode is ca. 1.5 mg cm–2.
2.2. Cell Assembling and Electrochemical Measurements
NCM90||Li cells (CR2025 coin-type) with a Celgard 2400 separator and 100 μL of an electrolyte for each cell were assembled in an argon (Ar)-filled glovebox (Mikrouna Co., Ltd.) with water and oxygen contents less than 0.1 ppm. The cells were maintained at static state for 5 h at 30 or 60 °C before galvanostatic charge/discharge performance tests. The cycle life of cells was evaluated on a BTS-5 V 10 mA battery testing system (NEWARE Electronic Co., Ltd.) between 3.0 and 4.3 V (vs Li/Li+) at 0.1C for the starting three cycles and at 1C for the following cycles (1C = 180 mA g–1). The rate capability test was performed at increasing discharge current rates, ranging from 0.5, 1, 2, 3, 5, and 7 to 10C with same 0.2C for charge, after three formation cycles at 0.1C. Linear sweep voltammetry (LSV) was carried out on a CHI760D (Chenhua, Shanghai) electrochemical workstation with a Pt working electrode at a sweeping rate of 0.1 mV s–1. CHI760D was also utilized to conduct cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). CV was implemented at a scan rate of 0.1 mV s–1 between 3.0 and 4.3 V for an NCM90 electrode, while EIS was measured from 100 kHz to 0.001 Hz at a charged state ∼4.2 V with a potential amplitude of ±5 mV.
2.3. Characterizations
To probe the morphological variation, the surface film thickness, and chemistry, the spent electrodes samples were investigated by scanning electron microscopy (SEM, ZEISS Sigma, Germany), transmission electron microscopy (TEM, JEM-2100HR, Japan), and X-ray photoelectron spectroscopy (XPS), respectively. Prior to the abovementioned characterizations, the electrode samples were soaked and thoroughly washed with dimethyl carbonate (DMC) to remove the residual electrolyte. The XPS experiment was conducted using a Quantum 2000 ESCA spectrometer (Physical Electronics) applying Al Kα radiation (1486.6 eV) for excitation under the vacuum of <10–8 Torr. XPS spectra were collected with a step size of 0.1 eV and calibrated by adjusting the strong C–C peak at 284.8 eV. X-ray diffraction (XRD) (Rigaku Ultima IV, Japan) was carried out using Cu Kα radiation (1.54056 Å) between 10 and 80° with a sweeping rate of 10° min–1 to analyze the crystal structure. The dissolving-out amounts of Ni, Mn, and Co from NCM90 were collected by inductively coupled plasma atomic emission spectroscopy (ICP-OES, SPECTROBLUE FMX36).
2.4. Theoretical Calculation Method
To calculate the HOMO/lowest unoccupied molecular orbital (LUMO) energies of the TBDB additive as well as of EC, DEC, and DMC, the geometric structures of TBDB, EC, DEC, and DMC were optimized by a B3LYP computing method, using the 6-311+G(d,p) basis set. (31) In the present study, for HOMO/LUMO energy level calculations, the solvent effect was considered using the solvation model based on density (SMD), and acetone (relative permittivity = 20.5) was selected as the solvent, which is slightly different from what has been used for our previous study (based on EC/EMC (relative permittivity ∼ 29)). (32)
3. Results and Discussion
3.1. Electrochemical Stability
To assess the electrochemical stability of TBDB and solvents, their HOMO and LUMO energies were first stimulated by theoretical calculations. Theoretically, the molecules with a higher HOMO energy level are more inclined to be oxidized at the cathode side, while those with a lower LUMO level indicate early reduction at the anode side. The DFT-calculated result demonstrates a narrow electrochemical window of TBDB. It can be found from Figure 2a that the HOMO energy value of TBDB is −7.60 eV, less negative in comparison to EC, DEC, and DMC which are −8.43, −8.09, and −8.20 eV, respectively, indicative of the priority of TBDB oxidization during the initial electrochemical charging process. At the same time, the lower LUMO energy value of TBDB (−0.41 eV), less than EC (−0.09 eV), DEC (−0.12 eV), and DMC (−0.11 eV), indicates a higher reduction potential of TBDB.

To testify the DFT-calculated results, LSV, CV, and dQ/dV were conducted to evaluate the oxidation and reduction potential for TBDB. As shown in Figure 2b, a distinct current response appears at around 5.8 V (vs Li/Li+) in the TBDB-containing electrolyte, which is about 0.5 V ahead of the baseline electrolyte (approximately 6.3 V vs Li/Li+), confirming that TBDB is prone to be oxidized prior to the carbonate solvents. This trend is further verified by the relatively lower onset oxidation potential as detected for the NCM90 cathode electrode (Figure S1). In addition, the dQ/dV profiles of the Si/C anode in the initial discharge process demonstrate that the reduction potential of TBDB is approximately 1.4 V (vs Li/Li+) (Figure S2), which is earlier than EC and DEC (∼0.7–0.8 V). The result suggests that TBDB might be a versatile additive for stabilizing both cathode and anode interfaces.
3.2. Electrochemical Performance
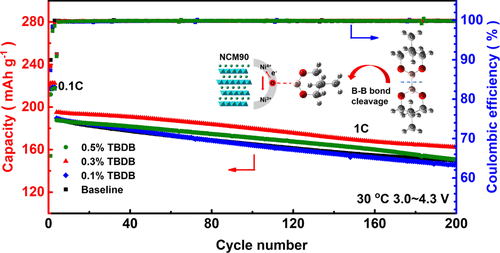
To understand the function of a TBDB additive on electrochemical performance, the NCM90||Li half cells were first evaluated at cutoff voltages between 3.0 and 4.3 V. As shown in Figure 3a, the NCM90||Li cells with the electrolyte containing an appropriate amount of TBDB exhibits improved cycling behavior than that with the base group. It is found that, after 200 cycles, the NCM90||Li cell using the baseline electrolyte delivers a discharge capacity of 149 mAh g–1 corresponding to a capacity retention of only 78.8%. In contrast, the cell using the 0.3% TBDB-containing electrolyte shows a better-reserved discharge capacity of 162 mAh g–1, corresponding to an enhanced capacity retention of 83.1%. In comparison, a lower content of 0.1% TBDB is insufficient to construct a stable CEI (Figure S3a), displaying similar cycling performance compared with the baseline electrolyte. On the other hand, a higher content of 0.5% TBDB might generate a thickened CEI, which is unfavorable for the prompt lithium-ion de/intercalation (Figure S3b). Excessively, addition of 1.0% TBDB leads to lower initial discharge capacity at both 0.1 and 1C, accompanied by accelerated capacity decay (only 84.2% retention after 90 cycles). Hence, based on the cycling performance, 0.3% TBDB is considered the optimized amount for the present study, and its functioning mechanism is systematically investigated. Figure 3b illustrates the first cycle charge/discharge profiles of NCM90||Li cells in the baseline electrolyte and the 0.3% TBDB-containing electrolyte. It is noted that the Coulombic efficiency in the 0.3% TBDB-modified electrolyte is 88.3%, marginally lower than that in the unmodified electrolyte (89.9%), which could be ascribed to the involvement of TBDB in the CEI formation process that contributes a bit of the irreversible capacity.

The average discharge voltages are further presented to compare the polarization evolution of electrodes (Figure 3c). The average discharge voltage of 3.84 V is preserved in the 0.3% TBDB-containing electrolyte after 200 cycles, which is higher than 3.81 V in the baseline electrolyte, indicating a better maintained structural/interfacial stability of the NCM90 cathode. Detailed charge/discharge curve evolution of NCM90||Li cells in baseline and TBDB-added electrolytes is demonstrated in Figure S4. Thanks to the improved interfacial structure stability benefited from the TBDB-derived interfacial layer on the NCM90 cathode, the charge/discharge profiles are better maintained. This conclusion could be further supported by analyzing the evolution of dQ/dV curves along cycling (Figure S5). As reported, the reversibility of H2–H3 phase transition (i.e., the redox peaks at about 4.1–4.2 V) could reflect the stability of a layered structure of a Ni-rich cathode. (33) The drastic shrinkage of redox peaks related to H2–H3 phase transition evidences the quickly degraded cathode surface layered structure. On the contrary, the reversibility of H2–H3 phase transition is largely ameliorated in a TBDB-added electrolyte, confirming the interfacial stabilizing effect in the presence of the TBDB additive. The improved structural stability is further evidenced by the better-preserved X-ray diffraction peaks of the NCM90 electrode after cycling in the electrolyte with the TBDB additive (Figure S6).
Elevating the charge cutoff voltage is generally adopted by industries and proved to be a useful measure to promote the energy density of LIBs. To satisfy the need for developing higher-energy-density LIBs, the high-voltage effectiveness of the TBDB additive was further corroborated, by charging the NCM90 to voltages of 4.4 or 4.5 V. As shown in Figure 3d and Figure 3e, by increasing the charge cutoff voltage to 4.4 or 4.5 V, NCM90 cathodes deliver higher initial discharge capacities of 224 and 230 mAh g–1 at 0.1C, respectively, which are much higher than that at a charge cutoff of 4.3 V (218 mAh g–1), indicating an obvious increase in energy density (from 843 W h kg–1 to 873 and 894 W h kg–1 calculated based on the cathode active material). However, the elevated charge cutoff voltage gives rise to more electrolyte decomposition at high voltages, causing expedited capacity degradation of LIBs. In the baseline electrolyte, the discharge capacity retention after 200 cycles is only 70.1 and 71.8% at 4.4 and 4.5 V, respectively, inferior to that at a 4.3 V cutoff (78.8%). As a sharp contrast, by introducing the TBDB additive, the discharge capacity retentions of NCM90||Li cells are markedly increased to 83.8% at 4.4 V and 81.6% at a charge cutoff of 4.5 V. The result substantiates the effectiveness of the TBDB additive and the derived CEI layer guarantees the decent interfacial stability of NCM90 even at high voltages. Beyond that, the positive impact of the TBDB additive is also proved by evaluating the NCM90 electrode with a high loading of about 7–9 mg cm–2 and graphite||NCM90 full cells. As shown in Figure S7, a thick NCM90 electrode exhibits a continuous capacity decay in the electrolyte without the TBDB additive, delivering 164 mAh g–1 with a capacity retention of 86.5% after 55 cycles. However, the thick NCM90 electrode in the electrolyte with 0.3% TBDB shows a clear capacity improvement, retaining a discharge capacity of 174 mAh g–1 with a higher capacity of 91.0% after same cycles. In addition, with the introduction of the TBDB additive, graphite||NCM90 full cells retain higher discharge capacity (180 mAh g–1) and higher capacity retention (97.0%) after 50 cycles versus that with the additive-free electrolyte (94.1%, 176 mAh g–1) (Figure S8). Since the TBDB additive could also be consumed for SEI layer formation on a graphite anode, the TBDB content needs to be further optimized for practical applications in large-format graphite||NCM90 full cells.
High-temperature stability of NCM90||Li cells was further evaluated at 60 °C to identify the effect of a TBDB additive at such a harsh condition (Figure 4a). The NCM90 electrodes in both the baseline electrolyte and the TBDB-containing electrolyte fade more rapidly at 60 °C than at 30 °C. The reason is ascribed to the fact that high temperature aggravates the parasitic side reactions at the cathode/electrolyte interface, devastating the electrode structural and interfacial stability, which accelerates the performance deterioration. In the baseline electrolyte, the NCM90||Li cell delivers an initial discharge capacity of 217 mAh g–1 at 1C, which degrades to 162 mAh g–1 after 100 cycles, equal to a capacity retention of 74.7%. In contrast, for the cell with the TBDB-added electrolyte, a superior discharge capacity of 171 mAh g–1 after 100 cycles is obtained with a better capacity retention of 78.8%. The improved cycling performance could be rationalized by the superior CEI layer formed in the TBDB-containing electrolyte, which protects the NCM90 cathode against electrolyte corrosion (from acidic species) at 60 °C.

Figure 4b shows the rate performances of NCM90 cells. The cells were subject to three cycles at 0.1C and then tested at C rates ranging from 0.5 to 10C. For the baseline group, the discharge capacities of NCM90 are 218, 203, 197, 190, 184, 177, 171, and 165 mAh g–1 at 0.1, 0.5, 1, 2, 3, 5, 7, and 10C, respectively (Figure 4c). In the TBDB-containing electrolyte, NCM90 shows superior rate capability, as evidenced by the higher discharge capacities at higher C rates. Remarkably, at an excessively high rate of 10C, the NCM90 electrode in the TBDB-added electrolyte retains a discharge capacity of 175, 10 mAh g–1 above that of the baseline counterpart (Figure 4d). Moreover, for the TBDB-containing electrolyte, a high capacity of 189 mAh g–1 is obtained when the current gets back to 1C, superior over the capacity of 173 mAh g–1 for the baseline electrolyte. Cycling performance evaluated at high C rates, i.e., 1C charge and 3C discharge (Figure S9), demonstrates that a fascinating capacity retention of 92.8% over 100 cycles could be achieved in the TBDB-containing electrolyte (versus to 86.6% in baseline electrolyte), further affirming the improved electrode kinetics with the optimized TBDB-containing electrolyte.
3.3. Electrochemical Impedance and Kinetics of the NCM90 Cathode
The EIS tests were implemented to reveal the evolution of electrochemical resistance in a baseline electrolyte and a TBDB-containing electrolyte (Figure 5). It can be detected that the impedance spectra include two semicircles accompanied by a straight line from a high to low frequency. The intercept at a high frequency is mainly corresponding to electrolyte resistance (Re), and the two semicircles are generally associated with the surface film resistance (Rsf) and charge-transfer resistance (Rct), while the straight line is related to the Warburg resistance (W0). The values of Re, Rsf, and Rct from the equivalent circuit (inset in Figure 5a) are exhibited in Table S2, and the evolutions of Rsf and Rct are illustrated in Figure 5c,d. In the baseline electrolyte, the total resistance (Re + Rsf + Rct) increases drastically to more than 400 Ω after 100 cycles (Figure 5a), which only increases to 230 Ω in the TBDB-containing electrolyte (Figure 5b). The faster resistance growth in the baseline electrolyte implies the continuous interaction between an NCM90 cathode and an electrolyte and the consequent accumulation of a massive and resistive interfacial layer. Compared to this, a robust CEI layer is created in the presence of the TBDB additive, inhibiting the exposure of the NCM90 cathode to the electrolyte and suppressing the increase of the interfacial resistance. Of note, the initial Rsf in the control electrolyte is greater than that in the TBDB-containing electrolyte, which could explain the slightly higher discharge capacity in the TBDB-containing electrolyte (Figure 3a) because lower resistance ensures faster lithium-ion diffusion. The decrease in Rsf in the first 50 cycles may be ascribed to the gradual stabilization process of the CEI film. (34) On the other hand, Rct, accounting for a primary part of total resistance, plays a decisive role in affecting electrochemical performance. The smaller and limited increase in Rct in the TBDB-added electrolyte (Figure 5d) provides solid evidence that the TBDB-derived CEI layer functions well to stabilize the NCM90/electrolyte interface.

To explore the effect of the TBDB additive on the lithium-ion diffusion kinetics, the Li+-ion diffusion coefficient of the NCM90 electrode was evaluated by the EIS method, (32) and is calculated by the following equations (35)

(1)

(2)where σ is the Warburg factor calculated by the linear relation between Zre and ω–1/2 at a low frequency; DLi+ is the Li+-ion diffusion coefficient; and A, R, T, n, F, and the C are the surface area of the electrode, the gas constant, the thermodynamic temperature, the number of electrons transferred, the Faraday constant, and the concentration of lithium ions, respectively. Figure 6 illustrates the linear relationship between Zre and ω–1/2 at the selected cycle, and the calculated Li+- ion diffusion coefficient values are listed in Table 1 according to eq 2. In the TBDB-containing electrolyte, the Li+-ion diffusion coefficient DLi+ is 1.5 × 10–9 cm2 s–1 in the initial cycle, about fourfold that (3.4 × 10–10 cm2 s–1) obtained in the baseline electrolyte. The enhanced diffusion kinetics is conducive to more Li+-ion utilization, and hence achieving slightly higher initial discharge capacities at 0.1 and 1C in the 0.3% TBDB-containing electrolyte (Figure 3a). In addition, higher diffusion kinetics using the TBDB-containing electrolyte is maintained after 100 cycles, which benefits the cycling and rate performance.

Table 1. Calculated Li+-Ion Diffusion Coefficient DLi+ of NCM90||Li Cells at Selected Cycles
| cycle no. | baseline (cm2 s–1) | 0.3% TBDB (cm2 s–1) |
|---|---|---|
| 1st | 3.4 × 10–10 | 1.5 × 10–9 |
| 4th | 2.5 × 10–10 | 4.4 × 10–9 |
| 10th | 1.2 × 10–10 | 1.8 × 10–10 |
| 50th | 1.8 × 10–10 | 1.7 × 10–10 |
| 100th | 7.2 × 10–11 | 1.2 × 10–10 |
3.4. Interfacial Morphology and Component Evolution of the NCM90 Cathode
The morphologies of the NCM90 electrode before and after 200 cycles were observed by SEM. To certify the active effect of TBDB, the NCM90 electrodes were tested at a high charge termination voltage of 4.5 V. As shown in Figure 7a–c, a fresh NCM90 particle surface is smooth and clean. After 200 cycles in the baseline electrolyte, the NCM90 particles are masked by a thick layer of byproducts originated from the decomposition of the electrolyte. In contrast, the NCM90 particles cycled in the 0.3% TBDB-containing electrolyte are still smooth with a well-defined particle surface, implying significantly prevented side reactions. TEM was further employed to reveal the microscopic difference of the CEI layer on the electrode interface (Figure 7d–f). TEM images clearly show that a loose and thick CEI layer of about 20 nm is built on the NCM90 cathode in the control electrolyte. However, with the addition of TBDB, a compact and thin CEI layer of only a few nm (7–8 nm) is constructed (Figure 7f). After 200 cycles in the control electrolyte, NCM90 particles are covered by a layer of byproducts with a thickness of 20–30 nm resulted from the decomposition of the electrolyte (Figure S10a,c). In contrast, the NCM90 particles cycled in the 0.3% TBDB-containing electrolyte are still smooth with a well-maintained particle surface covered with a thinner (∼15 nm) surface film, suggesting significantly prevented side reactions (Figure S10b,d). These microscopic analyses prove that the NCM90 surface could be well protected by a more robust CEI produced in the TBDB-containing electrolyte, therefore impeding the continuous electrolyte decomposition issues. Moreover, as a result of the enhanced interfacial stability, the dissolution of transition metal (Ni, Co, Mn) ions could also be obviously restrained, as confirmed by the ICP result (Figure S11).

The surface film composition on the NCM90 surface with and without a TBDB additive after three-cycle formation process was characterized by XPS (Figure 8). For the spectra of C 1s (Figure 8a,e), the strong peak at 284.8 eV could be ascribed to the C–C peak, while the other two peaks located at 286.3 and 290.9 eV are related to C–O and Li2CO3/RCO3Li. (36) In the O 1s spectra (Figure 8b,f), three peaks centered at 533.3, 532.5, and 531.3 eV could be indexed to C–O, B–O, and Li2CO3/lithium alkyl carbonate (C═O) species, respectively. Relatively higher intensity of the shoulder peak located at 531.3 eV is observed in the baseline electrolyte, suggesting more byproducts from the decomposition of carbonate solvents. (37) This also hints that the decomposition of electrolyte solvents is obstructed in the TBDB-containing electrolyte. In the O 1s spectra, the presence of the B–O peak (532.6 eV), (38) simultaneously ascertained in B 1s spectra at 192.0 eV (Figure 8d,h), clearly confirms that TBDB is involved in CEI formation. In the F 1s spectra (Figure 8c,g), three peaks located at 688.4, 687.6, and 685.0 eV are identified, indicating the existence of PVDF, LixPOyFz, and the LiF component. (39,40) Especially, the LiF component, which is poorly conducting, is generally produced from the decomposition of LiPF6 and the attack by the trace content of acidic species (HF, etc.). It is found that relatively lower intensity of the LiF peak is noted in the TBDB-modified electrolyte, clarifying less LiPF6 salt decomposed, which is in accordance with the limited increase in charge transfer resistance and the better long-term cycling performance as discussed above.

3.5. Possible Decomposition Mechanism of TBDB
It is speculated that there might be two main possible pathways for bond cleavage of the TBDB additive: boron–boron bond (41) and boron–oxygen bond. (27) Here, the energies for boron–boron and boron–oxygen bond breaking are calculated based on the average value due to the symmetry of the molecular structure of TBDB. As shown in Figure 9, the energy required for the boron–boron bond breaking is 0.12 hartree (1 hartree = 2625.5 kJ mol–1), which is energetically more favorable than that for the boron–oxygen bond breaking (0.155 hartree), indicating that the TBDB additive presents higher probability to break from the boron–boron bond rather than the boron–oxygen bond. The calculated result is in good accordance with the results when the diboron compounds are applied to an organic synthesis reaction. (42) Meanwhile, it is reported that the Lewis acid–base adduct obtained subsequent to the diboron compound being attacked by oxygen or nitrogen nucleophiles has a strong ability to donate an electron, (43) which is considered beneficial for lowering the valence of transition metal ions, especially the oxidative Ni4+. As a result, the oxidative decomposition of the electrolyte could be largely inhibited, ensuring the better electrochemical performance of NCM90||Li cells.

In addition, for the sake of verifying the effect of TBDB on the lithium-metal anode, Li||Li and Li||Cu batteries were cycled with a deposited capacity of 1 mAh cm–2. As indicated in Figure S12, it is demonstrated that TBDB has a marginal effect on the Li electrodes, showing similar lithium plating/stripping Coulombic efficiency as compared to the additive-free electrolyte. In this regard, the boosted electrochemical performance of NCM90||Li cells could be primarily ascribed to the contribution in stabilizing the interface of the NCM90 cathode electrode.
4. Conclusions
A novel diboron additive TBDB has been systematically investigated for stabilizing the Ni-rich NCM90. With the optimized 0.3% TBDB-containing electrolyte, NCM90||Li cells show significantly improved cycling performance both at 30 and 60 °C, as well as at a charge cutoff voltage up to 4.5 V, which shows great potential for practical applications in energy-densified LIBs, especially for EVs. Meanwhile, by suppressing side reactions, the TBDB-derived CEI layer is beneficial for maintaining superior interfacial reaction kinetics of the NCM90 cathode, enhancing the rate capability of NCM90||Li cells. The ameliorated electrochemical performance could be rationalized by the fact that TBDB with high HOMO energy tends to be oxidized prior to electrolyte solvents, through boron–boron bond breaking, generating a compact, thin, and protective CEI passivation layer. The fundamental understanding of this work points out a direction for designing diboron-type functional additives to exploit high-energy-density and long-cycle-life LIBs using Ni-rich cathodes.
【Article link】
https://doi.org/10.1021/acsaem.1c01977