Jackeline Soto-Cruz More by Jackeline Soto-Cruz, Paolo Conejo-Valverde, Giovanni Sáenz-Arce, Hongjing Dou, and Oscar Rojas-Carrillo* Cite this: Langmuir 2021, 37, 11, 3446–3455 Publication Date:March 8, 2021
Abstract
Negatively charged liposomes accomplished both functions as a reducing and stabilizing agent in the synthesis of gold nanotriangles (GNTs). Liposomes are based on a mixture of phospholipids phosphatidylcholine/phosphoglycerol, and they were used as a template phase to perform the GNTs. The method was evaluated under different conditions such as temperature, reaction time, phosphoglycerol chain length, and precursor concentration. Isotropic and anisotropic gold nanoparticles are formed simultaneously during the synthesis. Therefore, by combining centrifugation and depletion flocculation strategies, the sample was concentrated in terms of GNTs from 15% crude to 80% by using sodium dodecyl sulfate (SDS). As a result, a green colored dispersion was obtained containing highly purified, well-defined, negatively charged GNTs, where the edge length of most particles is centered in the range of 60–80 nm with an average thickness of 7.8 ± 0.1 nm. By this purification process, it was possible to highly increase the yield in terms of GNTs. Other surfactants [cetyltrimethylammonium chloride (CTAC), hexadecyltrimethylammonium bromide (CTAB), Tween 20, and dodecyldimethylammonium bromide] were evaluated during the purification stage, and both CTAB and CTAC show similar results to those obtained by using SDS. These GNTs are potential candidates for future applications in molecular imaging, photothermal therapy, drug delivery, biosensing, and photodynamic therapy.
Introduction
The study of anisotropic gold nanoparticles (AGNPs), such as nanorods, (1) nanotriangles, (2−4) nanoshells, (5) nanocapsules, (6) and nanostars, (7,8) have been extensively investigated during the last decades, motivated by their remarkable optical and electrical properties, which depend mostly on the size, shape, surface chemistry, and the aggregation state. (9−12) Among the anisotropic nanoparticles, gold nanotriangles (GNTs) have garnered substantial interest because of their promising optical properties. Different strategies have been reported for synthesizing GNTs, including seed-mediated, (2,4) deposition, (2) vesicular template phase, (13,14) and microwave-assisted approaches. (15)
Nevertheless, these methodologies have reported shape yields below 30–40%, and the samples often show high polydispersity because size control is not precise enough as the control that currently exists in the synthesis of gold nanorods. (4) Furthermore, the actual green approaches consist of the use of tamarind leaf extract, (16) lemon grass extract, (17) leaf extract of Callistemon viminalis, (18) and the adoption of template phases. (19) In a previous report, Liebig et al., 2016, described a green process for obtaining GNTs of about 44 nm based on the template phase approach, using negatively charged vesicles, containing the surfactant dioctyl sodium sulfosuccinate (AOT) and Phospholipon produced between 30 and 33% GNTs. (19)
The low yield obtained, polydispersity, and byproducts limit the full utilization of the GNTs in diverse applications. The development of efficient and fast mechanisms to separate a specific shape or size is critical for refining gold nanoparticle (AuNPs) distribution. In this context, Scarabelli et al., 2014, obtained monodispersed GNTs after a purification process by the depletion-induced separation. (4) The synthetic method is based on the generation of cetyltrimethylammonium chloride (CTAC)-coated Au and a fast addition to a final growth solution. The yield in terms of GNTs with an edge length of about 50–150 nm is higher than 50%, which can be further increased up to 95% after the purification step by using CTAC micelles. (4)
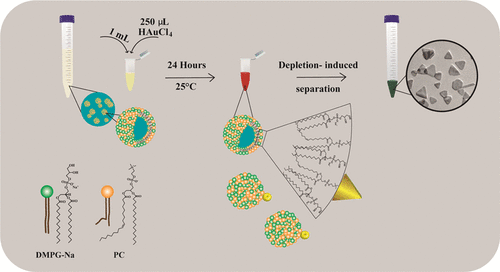
In the present work, we develop a robust and efficient strategy that combines the surface charge of the liposomes and their functional size to generate GNTs that were identified by an absorption band in the near infrared region (NIR), transmission electron microscopy (TEM), and atomic force microscopy (AFM). Our approach is inspired by the central role played by the liposomes as a template that directs the nanoparticle (NP) growth and the stabilization that is provided by the surface charge as well as their biocompatible, (20) bioadhesion, (21) and biodegradable (22) properties. Although extensive studies have emerged on the GNT synthesis exploiting the template phase method, (23,24) there is still no precise control on the NP shape. In consequence, synthetic reagents (sometimes, they might be very toxic) are used for shape and size control, limiting the biocompatibility of the final NPs. Therefore, the novelty of this project is the development of a green methodology by using biocompatible materials and avoiding the use of organic solvents or severe conditions to generate liposomes that can control the AuNP reducing and growth processes. This procedure was also developed under mild conditions in the absence of special media. These characteristics make the method desirable for large-scale NP synthesis or for exploiting the resulting NPs in biomedical applications. On the other hand, the GNTs were efficiently purified through a combined technique, applying centrifugation and the addition of sodium dodecyl sulfate (SDS) by the depletion flocculation mechanism. To the best of our knowledge, this is the first time that a refining approach is reported by using SDS. Additionally, the depletion stage was developed by using other surfactants.
Herein, we describe a synthesis for the fabrication of GNTs using nanoscale liposomes with intrinsic properties. The proposed methodology consists of l-α-phosphatidylcholine (PC) and dimyristoylphosphoglycerol sodium salt (DMPG-Na)-based vesicles that are negatively charged, which were previously characterized by Soto-Cruz et al., 2018. (25) We also show the effect of different conditions (temperature, reaction time, phosphoglycerol chain length, and precursor concentration) on the proposed strategy. A new method of purification was also developed, providing good performance in the separation of GNTs by using centrifugation and depletion interaction with SDS structures. Additionally, the refining procedure was compared by using different surfactants. These results contribute to the advancement of biomaterials with tremendous potential in applications like molecular imaging, (26) photothermal therapy, (27) diagnosis, (28) gen (29)/drug (30) delivery, biosensing, (31,32) and photodynamic therapy. (33)
Experimental Section
Materials
Gold(III) chloride trihydrate (HAuCl4·3H2O) (99.9%), dodecyldimethylammonium bromide (DDAB) (98%), and hexadecyltrimethyl ammonium chloride (CTAC) (98%) were purchased from Sigma-Aldrich. l-α-PC extracted from soy bean (PC) (94%), dimyristoylphosphoglycerol sodium salt (DMPG-Na) (98%), distearoylphosphoglycerol sodium salt (DSPG-NA) (98%), and dipalmitoylphosphoglycerol sodium salt (DPPG-Na) (98%) were donated by the company LIPOID (Germany). Hexadecyltrimethylammonium bromide (CTAB) (99%) was procured from Fisher Scientific. Polyoxyethylene (20) sorbitan monolaurate (Tween 20), SDS (99%) and absolute ethanol were acquired from J. T. Baker. All solutions were prepared using type I water (18.2 MΩ) provided by a Millipore Milli-Q water purification system.
Synthesis of GNTs
Vesicles based on the PC and phosphoglycerols were prepared following a procedure reported by Soto-Cruz et al., 2018. (25) Briefly, the hydration process was developed by dispersing 0.02 g of PC and 0.02 g of DMPG-Na in 20 mL of water type I. Afterward, the dispersion was stirred at 150 rpm for 72 h at room temperature. After that, 20 mL of water was added, and the dispersion was stirred for 72 h to obtain a final concentration of phospholipids of 1 mg/mL (0.5 mg/mL each phospholipid). This step was repeated for the PC/DPPG-Na and PC/DSPG-Na mixtures. In addition, PC dispersion (0.5 mg/mL) was prepared following the same method. Dispersions were stored at 4 °C until use. Subsequently, 5 mL were taken from the phospholipid dispersion, placed in a cold water bath, and ultrasonicated at 100 of amplitude for 1 min.
GNTs were prepared by mixing 1 mL from the phospholipid dispersion already sonicated with 250 μL of freshly prepared aqueous 2 mM tetrachloroaurate precursor solution (HAuCl4). The final concentration of the gold salt in the sonicated suspension was 0.4 mM. This parameter varied from 0.1 to 1.6 mM by adding 250 μL from the freshly prepared precursor solution at different concentrations in order to investigate its effect. The respective weight and molar ratio variations are available in Table S1. Mixtures were gently stirred at 120 rpm at 25 °C during 24 h in a water bath. This stage was continuously monitored by UV–vis spectroscopy. Different temperatures (15, 25, and 35 °C) were tested for the sample developed with the PC/DMPG-Na template phase, so as to find ideal conditions for GNT size and yield control. Afterward, samples were kept static at 25 °C during three months, and their stability was monitored by UV–vis spectroscopy at regular time intervals.
Purification of GNTs
A separation method based on centrifugation and depletion flocculation was performed using the AuNPs obtained by employing PC/DMPG-Na as a template phase. In 15 mL test tubes, eight samples of AuNPs (10 mL) were centrifuged at 4400 rpm for 1 h during two cycles. The supernatant was removed and the precipitate was dispersed with water until 10 mL after the first cycle of centrifugation and until 5 mL after the second cycle. Then, different surfactants solutions were prepared in water at specific concentrations [DDAB (0.045 M), SDS (0.3 M), CTAC (0.3 M), Tween 20 (0.2 M), and CTAB (0.2 M)] to evaluate their effect on the purification process. In addition, the CTAB stock solution was heated up until 40 °C by using a water bath, and it was used until the crystals were fully dissolved. Subsequently, each micellar dispersion was added separately to the AuNPs already centrifugated. After the addition, the optimum micellar concentration in the mixture was found as follows: 0.15 M for SDS, CTAC, Tween 20, 0.12 M for CTAB, and 0.015 M for DDAB. After adding the micellar dispersion to the AuNPs, the sample was kept static for four days. Afterward, the supernatant was removed, and the precipitate was dispersed in water until 2 mL.
Characterization
UV–vis absorption measurements were performed with a spectrometer Evolution 220 (Thermo Fisher Scientific, US) in the wavelength range between 300 and 1100 nm; samples were placed in plastic cuvettes (path length, 1 cm). Zeta potential and hydrodynamic diameter data were acquired by using a Zetasizer Nano-ZS90 (Malvern Instruments, UK) with a 90° angle, He–Ne laser (λ = 633 nm, 4 mW) at 25 °C; each determination was carried out ten times. Samples were placed in polystyrene cuvettes for hydrodynamic measurements. The size and shape of AuNPs were determined by TEM. Specimens were dropped on copper grids. Upon air drying, the samples were viewed on a TEM H-7100 (Hitachi, Japan), at an acceleration voltage of 100 kV. For statistical evaluation more than 800 particles were analyzed using Image J software. GNT thickness was assessed by AFM using the AFM NX10 (Park Systems, Korea) in true noncontact mode. Rectangular cantilevers PPP-NCHR were used (nominal length 125 μm, tip radius 2 nm and spring constant 42 N/m) with a resonant frequency of 330 kHz. The raw AFM data obtained were processed using XEI. Samples were prepared by depositing a 10 μL drop onto freshly cleaved mica and allowed 24 h to fully dry.
Results and Discussion
GNT Synthesis
In the aqueous phase, bilayers consisting of amphiphilic molecules (phospholipids) tend to self-assemble in spherical structures known as liposomes or vesicles. These architectures are based on a phospholipid bilayer that forms a hollow structure enclosing aqueous solution surrounded by an aqueous environment. In this work, a hydration step followed by a high intensity ultrasonication was used for obtaining small unilamellar vesicles (SUVs). The characterization results, before and after the ultrasonication step by dynamic light scattering (DLS), zeta potential, and cryo-SEM, were reported by Soto-Cruz et al., 2018. (25)
Initially, a turbid dispersion was generated under the conditions described above, and after the ultrasonication, this turned transparent. DLS results showed a narrow particle size distribution and sizes lower than 300 nm after the ultrasonication process. (25) It was also observed that the vesicular template phases (PC/phosphoglycerols) exhibited a negative zeta potential probably because of the influence of the deprotonated oxygen atom present both as in phosphoglycerols and in PC. (25)
After mixing the liposome dispersion with the gold salt solution in a weight ratio 10:1, respectively, a change of color was observed from transparent to dark red (Figure S1), which became stronger as time progressed. This color is a characteristic of the gold reduction process from Au3+ to Au0. Theoretically, the shape and size of NPs can be controlled by limiting the reaction rate. The metal–surfactant complex reduces the reaction rate even under highly favorable conditions. (34) In this research, phospholipids play an important role controlling the gold reduction, nucleation, and growth processes of the AuNPs, as well as generating a biocompatible coating, as shown in Scheme S1. Some significant outcomes were found in the UV–vis spectra of the NPs, indicating that the vesicles have a great influence in the GNT synthesis.
UV–vis absorption spectra help to generate a general idea about the AuNP size and shape. This behavior is supported by Mie’s theory that established that the peak position is directly related to the particle size. (35,36) According to the literature, gold spherical particles with sizes between 4 and 50 nm show absorptions in the range of 520–570 nm. (37) Theoretically, the first sharper peak around 525 nm (Figure 1) could be assigned to spherical AuNPs. It is also possible to observe that the NPs prepared in the presence of PC/phosphoglycerols as a soft template phase show a broad second absorption peak in the NIR; this could be preliminarily attributed to AGNP formation. Specifically, GNTs exhibit a longitudinal absorption band between 700 and 1400 nm, which corresponds to the surface plasmon in plane. (13) This band is extremely important in the biomedical field because it can penetrate biological tissues, causing an absence of significant absorption to normal cells (minimal damage). As it can be seen in Figure 1, the UV–vis results suggest the formation of anisotropic and spherical AuNPs.

Furthermore, the AuNPs_PC/DMPG-Na sample was further characterized by TEM to gather more detailed information about the NP shape. Figure 2 shows the specimen micrograph in which both triangular and spherical particles are observed. In other micrographs, hexagonal and rod structures were also found (Figure S2). Figure 2 also shows histograms of absolute frequencies of triangular and spherical particles, it required counting a total of 873 particles, where 15% of them are triangular structures and the remaining are spherical. According to Figure 2, the average edge length of the triangular NPs is 75 nm, while the average diameter of the spherical particles is 20 nm.

In the AuNP synthesis, each substance plays an essential role during the nucleation and growth stages. The sample that was developed only by using PC liposomes, showed the same change of color as those obtained by using the phospholipid mixtures; this indicates that PC can reduce the gold salt by itself. According to Figure 1, just one peak at 525 nm was found when PC liposomes were used, indicating the formation of gold spherical NPs. In contrast, phosphoglycerols are not able to reduce the gold salt by themselves because there is not a change of color in the solution. However, when these molecules are mixed with PC, an additional absorption peak could be observed on the NIR.
In addition, it is possible to observe in Figure 1 that the second band shifts to longer wavelengths when the phosphoglycerol chain length decreases. The AuNPs_PC/DMPG-Na sample has the second band at longer wavelengths with a well-defined absorption in comparison with the other samples. These differences found in the UV–vis spectra can be influenced by the size and shape of the final NPs, and these characteristics depend on the stabilization provided by the template phase during the synthesis process. Therefore, the higher negative charge might cause a better stability on the gold crystal surface. In this regard, liposome characterization developed by Soto-Cruz et al., 2018, showed that the zeta potential of the PC/DMPG-Na mixture was significantly more negative than the PC liposomes but in the same order than PC/phosphoglycerol mixtures. (25) These results have a similar trend to the AuNPs obtained using PC and PC/phosphoglycerol liposomes (Table 1). In consequence, NPs synthesized in the presence of liposomes based on PC/phosphoglycerols would be more favored because they might have a better stabilization (negatively charged liposomes), and this might influence the anisotropic growth of the crystal. Moreover, the adsorption of these molecules at the surface of the metal NPs provides high stability with time, remaining in dispersion without any evidence of aggregation (Figure S4).
Table 1. Characterization of AuNPs by DLS and Zeta Potential after the Synthesis Process
| samples | zeta potential (mV) | hydrodynamic diameter (d·nm)a |
|---|---|---|
| AuNPs_PC_0,5 mg/mL | 18 ± 5 | 277 ± 131 |
| AuNPs_PC/DMPG-Na | –56 ± 16 | 77 ± 28 |
| AuNPs_PC/DPPG-Na | –37 ± 11 | 48 ± 3 |
| AuNPs_PC/DSPG-Na | –44 ± 12 | 622 ± 40 |
a
Hydrodynamic diameter determined by DLS using the maximum intensity, representing the majority of the particles.
Besides, the electrostatic stabilization could take place by means of the phosphate groups because of their negatively charged density (oxygen atoms deprotonated) that could generate an electrostatic interaction with the surface of the NP. Similarly, Park and Shumaker-Parry, 2014, explained that in the stabilization of the synthesized NPs with sodium citrate the electrostatic interactions play a determining role. (38) In this context, the authors explained that the deprotonated oxygen in the citrate molecule could interact with the surface of the Au0 nuclei, orienting the growth of anisotropic AuNPs. (38) They also demonstrated that this adsorption (negative charge molecule-NP) is more favored on the Au {111} faces, leading the nucleation and growth steps. (38)
Additionally, Köth et al., 2012, proposed a possible mechanism for the GNT formation in the aqueous phase by the preferential absorption of vesicles on the crystal facets. (23) The authors argued that first crystalline seeds are formed. Then, the nuclei continue growing into thin plates, whose top and bottom surfaces are composed by {111} facets. The plate side probably consists of a combination of {100} and {111} facets because there is strong evidence that hexagonal plates are preliminarily formed before obtaining the GNTs. The final nanotriangles are obtained by the elimination of {100} sides because this facet has an energetic advantage in comparison with the {111} side. Thus, in the hexagonal plate, some facets grow faster than the other ones. (23) Based on the formation of triangular nanoplates from hexagons, it would be possible to think that this growing mechanism could be occurring in the present work because nano-hexagons were also found in the analyzed samples (Figure S2). However, further experiments are needed to confirm the growing process. Hong et al., 2011, and Shankar et al., 2005, suggested that the absorption of molecules in certain facets of the crystal favors the growth of a particular morphology. (39,40) Similarly, Li et al., 2006, and Lofton and Sigmund, 2005, support this mechanism from electron diffraction experiments on the nanocrystal. (41,42) In this context, Liebig et al., 2016, have demonstrated that the template phase (AOT)/phospholipid can bind selectively to {111} facets, reducing the growth rate in the vertical direction, and the growth in the lateral direction proceed via an Ostwald ripening growth mechanism. (13)
The AuNPs_PC/DMPG-Na synthesis was studied in more detail by UV–vis spectroscopy (Figures 3 and S3) as a function of time and temperature. Results show that the reaction temperature exerts control on the AuNPs (size and shape) and strongly affects the optical properties of the final NPs. In the outcomes obtained at 15 °C, only one absorption band at 525 nm was recorded (Figure S3A) which suggests the formation of spherical AuNPs. This can be explained because the liposomes might accumulate in all directions on the seeds, inducing equal growth, generating NPs with the same spatial distribution on all the axes.

Moreover, when an intermediate temperature (25 °C) is used, the maximum intensity in the NIR is achieved. The time-dependent UV–vis absorption spectra (Figure S3B) shows that initially, there is just one peak at 525 nm that corresponds to the spherical particles, which are probably used like crystal nuclei for the anisotropic NP formation. After 2 h, a second peak appeared at 1050 nm, which can be attributed to the formation of anisotropic NPs. The band in the NIR shifted toward shorter wavelengths after 4 h of reaction probably because of the redefinition of the GNT tips. Both peaks increased over time simultaneously after 2 h, this can be explained by an isochronous formation of the isotropic spherical and anisotropic NPs. Similar results were found by Liebig et al., 2016, in the GNT synthesis; these authors used a template phase based on AOT and phospholipids. (19) They observed the appearance of one peak in the UV–vis trace spectra around 525 nm after 24 min of the reaction, and a second peak in the NIR after 26 min. (19) This behavior, found at 15 and at 25 °C, could indicate that the nonpreferred structures require higher temperatures for achieving the activation energy in comparison with the spherical ones that are more energetically favorable. (14,43)
On the other hand, when the temperature increases up to 35 °C, two absorption bands can be observed after 1 h of reaction (Figure S3C), denoting that the nucleation stage has started earlier. After 24 h, the peak in the NIR is not as high as the band obtained at 25 °C (Figure 3). These outcomes suggest that the added heat accelerates the Au3+ reduction process; this probably leads to an enhanced nucleation rate and chloroaurate ions are consumed in the formation of nuclei, causing a reduction of available ions for the growth process. When the temperature rises, there are more collisions between molecules and ions; consequently, the control of the growth is lost. On the contrary, an intermediate temperature allows an adequate control over the growth of GNTs because of liposome adsorption in some specific facets, directing crystallization in the lateral direction. Similarly, Schulze and Koetz, 2017, found that the synthesis of GNTs using AOT, PC, and polyamplolyte PalPhBisCarb as a template phase showed a temperature and yield dependence. The authors observed a higher yield in terms of GNTs under intermediate conditions (45 °C). (14)
Furthermore, the effect of the gold(III) concentration was evaluated on the AuNPs_PC/DMPG-Na synthesis at 25 °C, and the UV–vis spectra were recorded after 24 h (Figure 4). For this experiment, the final gold salt concentration, after adding the solution to the liposomes, varied from 0.1 to 1.6 mM (Table S1). In Figure 4, one peak is observed between 500 and 600 nm, when 0.1 and 0.2 mM concentrations of the gold salt are used. The peak can be assigned to spherical AuNPs generated by the low precursor/liposome molar ratio due to liposomes and can easily stabilize every facet of the AuNPs, thus yielding spherical NPs. It is also possible to note that the intensity increases with the precursor concentration, this indicates that the gold salt is the limiting reagent of the reaction. The increase in the peak intensity with the precursor concentration is normally correlated with AuNP concentration. (44,45)

Additionally, when 0.4 mM gold salt concentration was used, two very well-defined maximums of absorption are obtained in Figure 4, suggesting the formation of spherical and anisotropic NPs as it was shown in TEM micrographs (Figure 2). This is possibly because of the adequate concentration of liposomes that allows the AuNP stabilization in some specific facets. When the gold salt concentration was increased up to 0.8 mM, the peak in the infrared range shifts to a shorter wavelength. When the precursor concentration is even higher (1.6 mM), the maximum of absorption in the NIR totally disappears. This probably occurs because of the unavailability of a sufficient amount of liposomes for stabilizing the AuNPs; therefore, spherical NPs are produced which stands for the reaction being highly dependent on the precursor concentration.
Purification of GNTs
Up to this point, there was still a considerable amount of byproduct particles in the sample after the synthesis. Therefore, two different strategies were combined for purifying GNTs; centrifugation and depletion flocculation.
After centrifugation, the sedimentation approach was applied, and different surfactants were evaluated in this stage. This phenomenon was proposed in 1954 by Asakura and Oosawa who demonstrated that the attraction between colloids increases in the presence of a free polymer or a surfactant above the critical concentration. (46) In the present work, the surfactant concentration was kept higher than the critical micellar concentration (cmc) and these dispersions were characterized by DLS and zeta potential (Table S2). The optimal surfactant concentration was found by evaluating the concentration causing a significant decrease of the band around 525 nm, as followed by UV–vis. During the flocculation step, a brown precipitate and a green re-dispersed solution were observed which is a characteristic of the GNT presence (Figure S5). Samples are analyzed by UV-VIS spectroscopy, and the result is available in Figure 5. As it can be seen, ionic surfactants show a better performance in the purification process in comparison with the nonionic because of the effective removal of the first band assigned to spherical NPs. Moreover, the intensity of the band in the NIR increased significantly, indicating the increment of GNTs in solution. The most efficient surfactants in the particle separation process, according to Figure 5, were SDS, CTAC, and CTAB. On the other hand, the NPs purified with Tween 20 and DDAB still show a band around 525 nm, evidencing that sub-products still remain in the solution. Besides, the sample that was purified with Tween 20 showed a decay in intensity on the band in the NIR, suggesting the GNT loss during the separation process.

According to Liebig et al., 2016, the depletion flocculation can be achieved by the depletion interactions between the NPs in the presence of micelles or polymers. (19) This kind of interaction is feasible in colloidal particles, and when the separation distance between the two NPs is smaller than the micelle diameter, a local concentration gradient (micelle concentration around the NPs is higher than between them) is achieved. Consequently, the solvent tends to diffuse where the micelles are, leading to an attraction between the two particles, inducing the flocculation. (47)
In particular, Figure 5 suggests that the addition of surfactant solutions to the AuNP samples, above the cmc in all cases, induces attractive interactions between the colloids and subsequently favored the flocculation of the GNTs, whereas other shapes (spherical NPs) remain in solution. GNTs possess a relatively large surface area, leading to large interactions as compared with the strong curvature surface in the gold nanospheres. (48) In consequence, the GNTs might exhibit a higher association rate than gold nanospheres, allowing a selective separation process. (49−51) On the other hand, DDAB swells in water to form two lamellar phases. (52) Typically, in dilute solutions (<0.06 M), a biphasic solution consisting of swollen lamellar crystallites have been determined. (53) This morphology probably does not create the sufficient attractive force, and strong depletion interactions cannot be efficiently achieved among GNTs. Finally, similar behavior was observed for the system purified with Tween 20, which can be explained in terms of partial adsorption on the NPs, generating a less efficient separation.
In addition, data on zeta potential and particle size of the purified AuNPs are shown in Table 2. DLS data show that the purified NPs ranged in size between 74 and 164 nm. Particularly, the refined sample using SDS shows negative zeta potential of the same order of magnitude as the SDS micelles, which suggests the adsorption of the surfactant molecules on the AuNP surface. This effect seems to be more evident in the purified samples with cationic surfactants, in which a charge inversion can be noticed. Moreover, the particles purified with Tween 20 show a decrease in zeta potential, which might be explained by the partial substitution of the phospholipid shell with the surfactant at the NP gold surface.
Table 2. Characterization of AuNPs by DLS and Zeta Potential after the Purification Process
| samples | zeta potential (mV) | hydrodynamic diameter (d·nm)a |
|---|---|---|
| AuNPs_SDS | –52 ± 9 | 74 ± 35 |
| AuNPs_CTAB | 45 ± 15 | 106 ± 47 |
| AuNPs_CTAC | 55 ± 6 | 164 ± 104 |
| AuNPs_DDAB | 65 ± 17 | 138 ± 95 |
| AuNPs_Tween 20 | –17 ± 8 | 98 ± 19 |
a
Hydrodynamic diameter determined by DLS using the maximum intensity, representing the majority of the particles.
Refined NPs by using SDS were further characterized by TEM (Figure 6); well-defined and rounded tips were identified. Moreover, it is possible to distinguish spherical, tubular, and other particles. Figure 6 also shows histograms of absolute frequencies ascribed to spherical and triangular NPs. It required counting a total of 922 particles, where 80% of them were triangular structures, and the remaining were spherical. According to Figure 6, the average edge length of the triangular particles is 70 nm, while the average diameter of the spherical particles is 60 nm. The average length obtained by TEM agrees with the size found by DLS (Table 2). The results show that the number fraction of nanotriangles increased drastically from 15% for raw, to 80% for the insolated products, therefore demonstrating the effectiveness of the depletion flocculation strategy.

Similarly, Park et al., 2010, achieved an efficient separation between nanorods, using a binary mixture of stabilizers (CTAB/BDAC), and AuNPs produced by using 0.225 M surfactant micelles made out 0.1 M CTAB, and 0.125 M BDAC. The fraction of rods increased from 73 to ∼99.9% after one sedimentation step. (47) In this context, Liebig et al., 2016, also showed that the initial yield in nanotriangles of 33%, produced by using a template phase, could be increased up to 99% by a combined polyelectrolyte/micelle depletion flocculation in the presence of PalPhBisCarb/AOT micelles and alginic acid/AOT micelles. (19)
Finally, GNTs are further characterized by AFM, and the outcomes are shown in Figure 7. As can be seen, Figure 7A shows nanotriangles and some agglomerates, probably because of the deposition process. An AFM image of an isolated GNT is shown in Figure 7B, with a height profile over the nanotriangle (Figure 7C). The height profiles confirmed that the nanotriangles are nanoplates, showing an average thickness of 7.8 ± 0.1 nm.

Conclusions
A one-step synthesis of GNTs in a mixed PC/DMPG-Na vesicle phase without the presence of additional reducing components was developed. These NPs showed high stability with time. The second band in the UV–vis spectra shifted to longer wavelengths when the phosphoglycerol chain length decreases. GNT synthesis showed a dependence on time and temperature; the optimum condition was reached in 24 h and 25 °C, where the maximum of absorption in the NIR was found. This preparation also showed a high dependence on the precursor concentration, being 0.4 mM, the best concentration found for the GNT obtention. A combined centrifugation and depletion flocculation approach was used for the removal of the gold nanospheres. This purification process was evaluated by using different surfactant solutions (SDS, CTAC, CTAB, Tween 20, and DDAB), where SDS, CTAC, and CTAB showed a better performance. A green colored dispersion was obtained containing highly purified, well-defined, negatively charged GNTs, where the edge length of most particles is centered in the range of 60–80 nm with an average thickness of 7.8 ± 0.1 nm. According to TEM micrographs, by using SDS, it was possible to increase from 15 to 80% the GNT population after two centrifugations and one sedimentation process. These results indicate that it is possible to synthesize GNTs by using a new template phase method and to provide an efficient and selective GNT separation through centrifugation and depletion-induced flocculation.
【Article link】