Li YangLi Yang
State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan 430071, P. R. China
University of Chinese Academy of Sciences, Beijing 10049, P. R. China
More by Li Yang, Yangming Jiang, Xinmiao Liang*, Youyi Lei, Tianci Yuan, Haiyan Lu, Zhihong Liu*, Yuliang Cao*, and Jiwen Feng
Cite this: ACS Appl. Energy Mater. 2020, 3, 10, 10053–10060
Publication Date:September 11, 2020
Abstract

The limitations of conventional organic liquid electrolytes such as sodium dendrite growth, serious side reactions, and liquid leakage hinder the development of sodium–metal batteries (SMBs). In this work, a novel sodium–poly(tartaric acid)borate (NaPTAB) salt with low cost and environmental friendliness was synthesized by an aqueous phase synthesis method. NaPTAB was combined with poly(vinylidene fluoride)–hexafluoropropylene (PVDF–HFP) to form NaPTAB-SM, and then NaPTAB-SM was swelled in the PC solution to obtain a single sodium-ion conductor gel polymer electrolyte (GPE), denoted NaPTAB-SGPE. NaPTAB-SGPE has perfect thermal stability with an initial decomposition temperature of 345 °C, a satisfactory ionic conductivity of up to 0.94 × 10–4 S·cm–1 at room temperature, a wide electrochemical window as high as 5.2 V (vs Na+/Na) at 30 °C, and a high sodium-ion transference number of 0.91 at 60 °C. Except for these satisfying performances, the Na3V2(PO4)3/Na cells assembled with NaPTAB-SGPE present excellent charge–discharge performance and stable cycling capability at high temperatures (60 °C). They also exhibit superior cycling stability compared to the liquid electrolyte cells with 1 M NaClO4 (EC/PC, 1:1, v/v, and 5% fluoroethylene carbonate (FEC)). After cycling, NaF is generated on the polymer electrolyte membrane as observed by 23Na and 19F solid-state NMR, which is more likely responsible for the excellent charge–discharge stability and cycling performance of the battery. These results show that NaPTAB-SGPE is a great potential alternative for solid-state sodium batteries.
1. Introduction
ARTICLE SECTIONS
Rechargeable lithium batteries have been widely used in portable electronics and electric vehicles, but the shortage of lithium has hampered their large-scale grid energy storage. (1−3) Sodium batteries are widely considered as an attractive alternative and recently have been extensively studied because of low cost, abundant resources, and similar electrochemical behavior and energy storage principle to lithium. (4,5) In addition, a sodium–metal anode exhibits high specific capacity (1166 mAh·g–1) and the lowest redox potential (−2.71 V vs standard hydrogen electrode), which enable the construction of high-energy-density sodium–metal batteries (SMBs). (6) However, as in the case of Li-metal batteries, Na metal easily reacts with the conventional electrolyte and forms unstable solid electrolyte interphase (SEI), which leads to sodium dendrites and may cause safety hazards during long cycling. (7)
To promote the uniform deposition of sodium ions, several strategies have been proposed and adopted, mainly including Na anode protection including building various surface protective layers (8,9) and development of new electrolytes in different concentrations, (10) components, (11) or/and forms. (12) Among them, the latter way is considered to be effective for practical battery applications. The electrolyte is an important part of the battery, which is mainly divided into three types: liquid electrolyte, solid electrolyte, and gel polymer electrolyte (GPE). (13) In particular, electrolytes with satisfactory ionic conductivity, wide electrochemical window, high sodium-ion transference number, and ideal interface compatibility are not only conducive to interface stability but also meet the safety requirements for realizing high-energy-density sodium–metal batteries. (14,15) The conventional liquid electrolyte usually has high ionic conductivity. However, there are safety issues, such as serious side reactions and liquid leakage that will directly affect the development of batteries. (16) Some works have been done and reported. Cao et al. (10) reported a new electrolyte by increasing the concentration of sodium bis(fluorosulfonyl)imide (NaFSI) salt to stabilize the Na metal anode. The way is effective, but at the same time the high concentration and cost of the electrolyte also limit the practical applications. As new lithium salts, lithium bis(oxalato)borate (LiBOB) (17) and lithium-oxalyl difluoroborate (LiODFB), (18) sodium-difluoro(oxalato)borate (NaDFOB) (11) were also synthesized in the past few years. They have low cost, excellent thermal stability, and considerable lithium/sodium-ion transference numbers. Gao et al. (19) reported a new NaDFOB-based electrolyte, which exhibited satisfactory compatibility with the Na anode and enabled excellent performance in different SMBs. Besides, they also demonstrated that the excellent charge–discharge stability is attributed to the Na4B2O5, NaBF4, and NaF component of SEI in NaDFOB-based symmetric cells by solid-state NMR. However, the electrolyte still suffers from safety problems of liquid leakage owing to the existence of highly flammable organic electrolyte solvents (e.g., ethylene carbonate, dimethyl carbonate). Solid electrolytes have been reported to replace combustible organic liquid electrolytes and inhibit dendrite growth because of their inherent safety. (20,21) However, it is almost impossible for their low ionic conductivity (10–8–10–6 S·cm–1) to meet the commercial requirements of SMBs. (22,23) Therefore, it is indispensable to develop suitable GPEs to ensure high performance and safety of the battery. The traditional GPEs are usually dual-ion polymer electrolytes and obtained by soaking the polymer in liquid electrolytes. Yang et al. (24) prepared the main polymer poly(vinylidene fluoride)–hexafluoropropylene (PVDF–HFP) through a simple phase separation process and soaked it in 1 mol·L–1 NaClO4/(EC/DEC/DMC = 1:1:1, v/v/v). The ionic conductivity of the above GPEs reached 6 × 10–4 S·cm–1 at room temperature. However, concentration polarization would reduce the energy efficiency of the battery, caused by the opposite movement of anions and cations under an applied voltage. (25) A single-ion conductor polymer electrolyte (SIPE) fixes anions on the polymer skeleton and only cations can move freely. The cation migration number of SIPE is close to 1, whereas the traditional dual-ion polymer electrolytes have a low value of about 0.3. Thus, concentration polarization of SIPE can be avoided, and the recycling performance of the battery with SIPE also can be improved. (26) These unique characteristics provide SIPE with potential application in batteries. There have been some reports on lithium single-ion conductor polymer electrolytes in recent years but a few on sodium. For example, Wang et al. (27) reported a lithium SIPE based on a novel polymeric lithium salt, which exhibited excellent conductivity of 1.41 × 10–4 S·cm–1 at 20 °C and a high lithium-ion transference number of 0.93 at 80 °C. Wang et al. (28) reported a SIPE by combining poly(vinylene carbonate) with polymeric sodium salt with high ionic conductivity and sodium-ion transference number.
In this paper, a new type of sodium–poly(tartaric acid)borate salt (NaPTAB) with low price and environmental friendliness was facilely synthesized by water-phase synthesis with tartaric acid, which exhibited a polyanionic structure with a high concentration of sodium ions per unit and high decomposition temperature. Moreover, as a polymer matrix, PVDF–HFP with lower crystallinity is a favorable scaffold material. It has been widely studied for its corrosion resistance, high melting point, and excellent mechanical properties. Herein, we report a novel single-ion gel polymer electrolyte NaPTAB-SGPE, NaPTAB-SM swollen with PC, for SMBs based on PVDF–HFP. It was manifested that NaPTAB-SGPE exhibited excellent electrochemical stability and compatibility with sodium–metal electrodes, high ionic conductivity, and outstanding sodium-ion transference number. In addition, Na|NaPTAB-SGPE|Na3V2(PO4)3 (NVP) cells present pleasurable cycling stability and excellent multiplier performance at a high temperature of 60 °C. 23Na and 19F solid-state NMR were performed to unveil the underlying chemistry of the NaPTAB-SGPE polymer electrolyte during battery cycling.
2. Experimental Section
ARTICLE SECTIONS
2.1. Materials
Boric acid (H3BO3, 99.99%), sodium hydroxide monohydrate (NaOH), poly(vinylidene fluoride)–hexafluoropropylene (PVDF–HFP, MW = 400 000, PVDF/HFP = 78:22), and propylene carbonate (PC, 99.9%) were purchased from Sigma-Aldrich. Tartaric acid, cyclohexane, and N,N-dimethylformamide (DMF, 99.8%) were supplied by Sinopharm Chemical Reagent Co. Ltd. NaClO4 (1 M) in ethylene carbonate/propylene carbonate (EC/PC, 1:1, v/v) with 5 vol % fluoroethylene carbonate was purchased from DoDoChem. The above materials were used directly without further purification treatment.
2.2. Preparation of NaPTAB, NaPTAB-SM, and NaPTAB-SGPE
The polymer salt was synthesized by the aqueous phase synthesis method. Boric acid (0.05 mol) and sodium hydroxide (0.05 mol) were added to deionized water (50 mL) and stirred for 1 h, labeled as solution A. Tartaric acid (0.05 mol) was added to 50 mL of deionized water and stirred to dissolve completely, named solution B. Solution B was added dropwise to solution A and stirred for 6 h at 90–95 °C. After the reaction, cyclohexane was added for azeotropic water removal, and a rotary evaporator was used in vacuum until a white solid appeared. After 12 h of vacuum drying at 100 °C, water was further removed to obtain the polymer salt named NaPTAB.
The electrolyte membrane NaPTAB-SM was prepared by the doctor blading process, and the synthetic route is illustrated in Figure 1a. PVDF–HFP was dissolved in DMF and stirred to form a (17 wt %) homogeneous solution; then, NaPTAB powder (NaPTAB/PVDF–HFP = 1:1, wt %) was added. NaPTAB-SM was prepared by the doctor blading process, and the DMF in the solution was removed after vacuum drying at 100 °C for 24 h. NaPTAB-SGPE was finally obtained after soaking PC (NaPTAB-SM/PC = 1:2, wt %) for 2 h and removing extra solvent from the surface.

2.3. Material Characterizations
The structures of NaPTAB, NaPTAB-SM, and NaPTAB-SGPE were characterized by 1H NMR and 11B NMR spectra collected on a Bruker AV III 500 MHz spectrometer (11.7 T) with a BBO probe. The 1H chemical shifts were referenced to tetramethylsilane (TMS) (δ = 0 ppm), and 11B chemical shifts were H3BO3 (0.1 M a.q., δ = 19.6 ppm). The crystal forms were analyzed by X-ray diffraction (XRD, D8 ADVANCE) equipped with Cu Kα radiation and a Fourier transform infrared (FTIR, Nicolet iS5, Thermo Electron Corporation) spectroscope. The surface morphologies were investigated by scanning electron microscopy (SEM, Hitachi S-4800). We also tested thermal behavior by a thermogravimetric analyzer (TGA, Rubotherm-DYNTHERM-HP) from 35 to 600 °C at a heating rate of 10 °C·min–1 under a nitrogen atmosphere. The tensile properties of the membranes (length, 29 mm; width, 10–15 mm; thickness, 30–60 μm) were measured using a tensile tester (Instron 5967).
2.4. Electrochemical Measurements of NaPTAB-SGPE
The Na+ ionic conductivity of NaPTAB-SGPE was measured in a blocking battery via electrochemical impedance spectroscopy (EIS). The EIS results were characterized on an electrochemical workstation (CS350H, Wuhan CorrTest) under a condition of frequency range from 1 MHz to 1 Hz between 30 and 100 °C. The blocking battery was assembled in an Ar-filled glovebox (water content below 0.5 ppm) with a structure of SS|NaPTAB-SGPE|SS. The Na+ ionic conductivity (σNa+) was calculated from the equation

(1)where σ denotes the ionic conductivity, L is the thickness of the composite membrane, Rb is the bulk resistance, and S is the area of the stainless steel electrode with the same diameter as that of the electrolyte membrane.
The sodium-ion transference number (tNa+) of the NaPTAB-SGPE electrolyte was measured according to the method reported by Evans et al., (29) by placing the GPE membrane electrolyte between two sodium foils. The tNa+ is determined by the electrolyte resistances and the steady-state currents; thus, by combining EIS and direct current (DC) techniques, the value of tNa+ is obtained. The DC potential of 10 mV was performed with an electrochemical workstation (CS350H, Wuhan CorrTest). The battery was assembled with a structure of Na|NaPTAB-SGPE|Na, and the tNa+ is calculated as

(2)where Is and Io represent the currents at the steady and initial states, Ri and Rf are the initial and the steady-state body resistances, respectively, Ro and Rs are interface resistances, and ΔV is the DC potential applied across the cell.
The electrochemical window of GPE placed between SS and Na foil (Na|NaPTAB-SGPE|SS) was analyzed by linear sweep voltammetry (LSV) measurements at a scan rate of 1 mV·s–1 between 0 and 6 V (vs Na+/Na) at 30 and 60 °C.
The galvanostatic polarization tests of Na|NaPTAB-SGPE|Na symmetric cells were carried out at a current density of 50 μA·cm–2 and a capacity of 25 μAh·cm–2 at 60 °C on a LAND CT2001A battery test system (Wuhan LAND).
2.5. Battery Performance Measurements
CR2032 cells Na|NaPTAB-SGPE|Na3V2(PO4)3 were assembled. Na3V2(PO4)3 were synthesized based on the previous work. (30) Briefly, 0.04 mol oxalic acid and 0.01 mol V2O5 were dissolved in 100 mL of deionized water at 80 °C under magnetic stirring. Then, 0.03 mol NaH2PO4 and 0.006 mol glucose were added into the above solution. After stirring for 6 h, the obtained solution went through the spray-drying process at an inlet temperature of 200 °C and an outlet temperature of 110 °C. The obtained powder was pyrolyzed at 850 °C for 8 h at a heating rate of 2 °C min–1 under a nitrogen atmosphere to obtain the NVP materials. The cathode was prepared by the blend coating method through mixing NVP materials, acetylene black, and PVDF in a weight ratio of 80:10:10 to disperse in N-methyl pyrrolidone (NMP). The mixture was then coated on a piece of Al foil and dried in vacuum at 100 °C for 12 h. Finally, the dried Al foil was punched into disk-shaped electrodes and the loading of NVP materials was about 1.56 mg cm–2 (including the weight of acetylene black and PVDF). All assemblies of CR2032 coin cells were carried out in an argon atmosphere glovebox. The cyclic voltammetry (CV) test was examined on CS350H in the potential range between 2.5 and 3.8 V at a sweep rate of 1 mV·s–1. The cycling performance of cells was examined on the LAND CT2001A battery test system.
3. Results and Discussion
ARTICLE SECTIONS
3.1. Structure Characterizations
The polymer salt is synthesized by aqueous phase synthesis, which is simple and environmentally friendly. The preparation process of NaPTAB is shown in Scheme S1 (Supporting Information). We analyzed and determined the chemical structure of NaPTAB using 1H and 11B NMR spectra. The 1H NMR spectra are shown in Figure S1a. The signals of −OH and −COOH in NaPTAB using tartaric acid as the reaction raw material disappear after the reaction. Moreover, the −CH signal in NaPTAB shifts from 4.31 to 3.33 ppm, indicating that a polymeric borate is formed through the reaction of −OH and −COOH of boric acid. From the 11B NMR spectrum in Figure S1b, a 11B signal peak at 10.72 ppm further confirms the formation of the reactant and the product. In addition, there is only one 11B signal peak in the product, indicating that the polymeric borate has a single chemical structure.
The SEM images reveal the surface morphologies of NaPTAB particles and the NaPTAB-SM membrane. The surface of NaPTAB particles is dense and smooth, as shown in Figure 1b. Meanwhile, the NaPTAB-SM membrane has a uniform porous structure with a pore size of about 3 μm, as shown in Figure 1c,d, respectively. In addition, uniform films were prepared. It can be seen that after adding PC, the NaPTAB-SM electrolyte membrane changes from white to translucent, see Figure 1e,f. Moreover, the solvent-swelled GPE membrane NaPTAB-SGPE still exhibits excellent uniformity and quasi-solidness, as shown in Figure 1f. These can be further illustrated according to the cross section in Figure 1g, and the thickness is about 50 μm. The uniform pore structure and flexibility make it a potential candidate for the interface stability of sodium–metal batteries, as shown in Figure 1h.
From the FTIR results in Figure S2a for PVDF–HFP, the absorption peaks at 2960 and 3016 cm–1 belong to the symmetric and asymmetric stretching vibration peaks of −CH2 and that at 1400 cm–1 is the deformation vibration peak of −CH2. A pair of absorption peaks at 1210 and 1176 cm–1 mainly correspond to the stretching vibration of −CF2; the peak at 510 cm–1 is the deformation vibration peak of −CF2; the sharp absorption peaks at 1070, 975, 853, 795, 763, and 613 cm–1 belong to the vibration of the crystalline phase; the absorption band at 885, 840, 741, and 602 cm–1 are the characteristic absorption peaks of the amorphous phase. (31,32) After recombination shown in Figure S2b, the peak in the amorphous region is significantly enhanced and widened and the peak intensity in the crystallization region is significantly decreased, which are consistent with the XRD results. In addition, the expansion vibration peaks of −CH2 and −CF2 weaken with the addition of the NaPTAB polymer salt. We suspect that the fixation of the anions in NaPTAB with the polymer chain of PVDF–HFP causes the F atom to interact with the H atom of −CH2 again, reducing the polarity of −CF2 and weakening the interaction between the polymer molecular chain and the sodium ion, thus facilitating the migration of sodium ions.
The XRD results of the NaPTAB salt show an obvious amorphous phase pattern. Also, pure PVDF–HFP has a semicrystalline structure, as shown in Figure 2a, with distinct characteristic diffraction peaks at 2θ = 18.3, 20.0, 26.4, and 39.9°. (33) The addition of NaPTAB weakens the intensity of the diffraction peaks but does not change the position of these peaks, indicating that the addition of NaPTAB can significantly reduce the crystallinity of the polymer electrolyte membrane. These will accelerate the movement of the long polymer chain and thus improve the migration rate of sodium ions in it to improve the ionic conductivity.

As shown in Figure 2b, the thermogravimetric analysis (TGA) curves reveal that NaPTAB and NaPTAB-SM exhibit high thermal decomposition temperatures of about 338 and 345 °C, respectively, which could be the contribution of their stable polyanionic structures. Furthermore, it should be noticed that the initial decomposition temperature of the NaPTAB-SM membrane shows some hysteresis compared with that of NaPTAB, indicating that NaPTAB has been encapsulated within PVDF–HFP. In addition, with a further increase of temperature, NaPTAB-SM begins to show a second weight loss at about 440 °C, which is caused by the thermal decomposition of PVDF–HFP. (34) These excellent thermally stable properties indicate that the prepared NaPTAB polymer sodium salt and NaPTAB-SM polymer electrolytes exhibit better safety properties than the traditional liquid electrolytes that could cause combustion induced by the excessive battery temperatures, which makes them as promising candidates for applications in sodium–metal batteries operating at high temperatures.
An electrolyte membrane with good mechanical properties helps SMBs to establish stable interfaces and improve safety. The tensile tests were used to evaluate the mechanical properties of PVDF–HFP, NaPTAB-SM, and NaPTAB-SGPE membranes. The stress–strain curves are displayed in Figure S3. The elongation at break of NaPTAB-SM films (15.12%) is much greater (about 5 times) than that of PVDF–HFP (3.13%). As expected, the NaPTAB-SGPE membrane has satisfactory plasticity (173.07%), which is mainly due to the swelling effect of the PC solvent. As a plasticizer, PC has a plasticizing effect, which is distributed between large molecular chains, can reduce the intermolecular force, reduce the polymer viscosity, and enhance flexibility. Thus, NaPTAB-SGPE has greater elongation than that of PVDF–HFP and NaPTAB-SM. So, the great plasticity of NaPTAB-SGPE is helpful to the practical application of flexible SMBs.
3.2. Electrochemical Performance
Figure 3a shows the impedance results of NaPTAB-SGPE materials varying from 30 to 100 °C. Then, the Na+ ionic conductivities at variable temperatures are calculated from bulk resistances, as illustrated in Figure 3b. Initially, the conductivity is 0.94 × 10–4 S·cm–1 at 30 °C and reach 1.43 × 10–4 S·cm–1 at 60 °C, and low activation energy (Ea = 0.127 eV) is calculated according to the Arrhenius equation, which could be considered as a candidate GPE for application in SMBs.

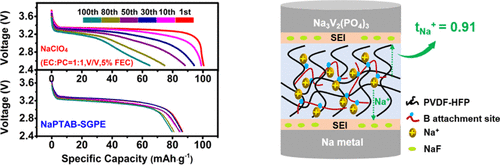
The Na+ transference number was measured according to the method of steady-state current. Using the data in Table S1 yield from Figure 3c, tNa+ turns out to be 0.91 according to eq 2, which is much higher than most dual-ion conductor electrolytes (0.3–0.5). This further verifies that the NaPTAB we prepared has a polyanionic structure, and the transport ions in the polymer electrolyte is almost only Na+ for bonding between large scale anions with PVDF-HFP polymer chainslimited thelong-distance movement. As shown in Figure 3d, NaPTAB-SGPE exhibits excellent electrochemical stability property for the wide electrochemical window. The initial potential of oxidation decomposition is up to 5.2 V, indicating that NaPTAB-SGPE has good electrochemical stability. Therefore, it could be suitable for extensive high-voltage electrode materials, (35) such as Na3V2(PO4)3 (voltage platform, ∼3.4 V), Na3V2(PO4)2F3 (voltage platform, ∼3.95 V), Na2CoPO4F (voltage platform, ∼4.3 V), etc. (36) These demonstrate that NaPTAB-SGPE is a promising electrolyte material for safe application in SMBs.
The interfacial stability is critical to the cycle life and safety of solid-state batteries. Polarization tests of Na symmetrical cells were performed with a constant current density of 50 μA·cm–2 at 60 °C to monitor sodium deposition/dissolution and study the interfacial stability between NaPTAB-SGPE and Na metal, as shown in Figure 3e. The polarization voltage reaches a stable level after 50 h, and there is no significant change in the following cycles. It indicates that the NaPTAB-SGPE film may effectively promote the deposition/dissolution of sodium, thereby inhibiting the growth of sodium dendrites.
3.3. Cell Performance
Full NVP/Na cells were assembled with NaPTAB-SGPE and the cycling performances were evaluated and are shown in Figure 4. As shown in Figure 4a, CV curves for the initial five cycles were recorded at a scan rate of 0.1 mV·s–1 in the voltage window of 2.5–3.8 V (vs Na+/Na). In the initial scan, the peaks appearing at about 3.6 and 3.2 V correspond to the stripping and planting of Na during the first charge–discharge cycle, respectively, which are consistent with the characteristic redox peaks of the NVP cathode. The five curves almost completely overlapped, suggesting that the NaPTAB-SGPE film has excellent stability and good compatibility with electrode materials during cell cycling.

Figure 4b displays the rate cycling performances of NaPTAB-SGPE for sodium–metal batteries under various current densities from 0.05C to 0.8C, exhibiting a consistently stable rate capacity at different charge–discharge rates. The cells display the discharge capacities of 90.3, 80.1, 67.3, 48.7, and 37.1 mAh·g–1 at 0.05C, 0.1C, 0.2C, 0.5C, and 0.8C, respectively. When the current density recovers to 0.05C, a specific capacity of 87.2 mAh·g–1 is acquired after 50 cycles.
The constant charge–discharge curves with different cycles and the specific capacity curves for NaPTAB-SGPE were tested within a voltage range from 2.5 to 3.8 V at a current density of 0.2C and are illustrated as Figure 4c,d, respectively. Obviously, we can see that these curves in Figure 4c have two voltage platforms at 3.6 and 3.2 V, which are consistent with the peaks of the CV curves, respectively. Related tests of the cells assembled with NaClO4 (EC/PC, 1:1, v/v, 5% FEC) as comparison are shown in Figure 5. Obviously, the GPE-based cells can achieve almost the same specific capacity as liquid electrolyte-based cells. The specific capacity of cells assembled with NaClO4 decay quickly and drop to 46.5 mAh·g–1 after 150 cycles (see Figure 5a), but the cells with NaPTAB-SGPE as the electrolyte still afford a high reversible capacity of 80 mAh·g–1 with little decay. Besides, as shown in Figure 5b, the discharge curves of batteries assembled with NaPTAB-SGPE are more concentrated than those of NaClO4 at different cycles. These results fully prove that the NaPTAB-SGPE gel electrolyte has excellent cycling stability. This might be because a stable SEI interface is formed between electrodes and the electrolyte. Such a stable rate performance is likely attributed to the fast Na-ion diffusion in NaPTAB-SGPE and the excellent structural stability with no side reactions of NaPTAB when the SEI interface formed compared to traditional liquid electrolyte. Figure 4e displays the cycling performance of NaPTAB-SGPE as the electrolyte of SMBs at 0.5C. After 500 cycles, a capacity of 45 mAh·g–1 can remained and the Coulombic efficiency is still close to 100% (>98%), suggesting excellent cycling stability of NaPTAB-SGPE. The related comparison with other solid-state sodium–metal batteries based on various polymer-based electrolytes and cathodes/anodes is shown in Table 1. These results certificate that NaPTAB-SGPE is a good candidate electrolyte material for SMBs compatible with the NVP or other cathodes to address the safety issues by alternating the organic liquid electrolyte to our gel polymer electrolyte.

Table 1. Related Comparison with Other Solid-State Sodium–Metal Batteries Based on Various Polymer-Based Electrolytes (37−39) and Cathodes/Anodes
| cathodes/anodes | average capacity retention rate (%) | current density | cycle number | polymer electrolyte | references |
|---|---|---|---|---|---|
| Y-doped Na2ZrO | 0.29 | 0.5C | 100 | NaClO4 + PEO | (39) |
| Na0.66Ni0.33Mn0.67O | 0.80 | 0.2C | 50 | NaTFSI + PEO | (20) |
| Na3V2PO4 | 0.86 | 0.5C | 50 | NaPA + PVDF–HFP | (37) |
| Na3V2PO4 | 0.94 | 0.5C | 50 | PSTB + poly(vinylene carbonate) | (28) |
| hard carbon | 0.83 | 0.5C | 100 | NaClO4 + PVDF–HFP | (38) |
| Na3V2PO4 | 0.97 | 0.2C | 150 | NaPTAB-SGPE | this work |
| 0.90 | 0.5C | 100 | |||
| 0.72 | 0.5C | 500 |
To understand why this NaPTAB-SGPE polymer electrolyte exhibits these excellent electrochemical performances, we collected NaPTAB-SGPE from the NVP/Na cells (after 150 cycles at 0.2C) in an Ar-filled glovebox (water content below 0.5 ppm) and performed 23Na and 19F MAS NMR experiments to probe the underlying chemistry of this material. The 23Na MAS NMR spectra were obtained on a Bruker 500 MHz (11.7 T) solid-state NMR spectrometer with a 4 mm HXY MAS probe at a speed of 10 kHz, and the 19F MAS NMR spectra were obtained on a Bruker 400 MHz (9.4 T) solid-state NMR spectrometer with a 4 mm HFX MAS probe at a speed of 10 kHz. Furthermore, the 23Na chemical shifts were referenced to NaCl (1 M a.q., δ = 0 ppm) and the 19F chemical shifts were referenced to solid ammonium trifluoroacetate (CF3COONH4, δ = −75.5 ppm). Figure 6 displays the 23Na (a) and 19F (b) MAS NMR results of NaPTAB, NaPTAB-SM, and NaPTAB-SGPE before and after battery cycling. From the 23Na NMR results, the NaPTAB signal at −12.3 ppm shifts to −7.7 ppm in NaPTAB-SM, and the line width is obviously narrowed, indicating higher mobility of Na ions in NaPTAB-SM than that in NaPTAB. After 150 cycles, the peak broadens again and shifts to a high field, and two new peaks at 7.10 ppm and 19.7 ppm emerge, revealing that new components are generated during cycling. The weak signal at 19.7 ppm is assigned to the −ONa structure. The 7.10 ppm peak is attributed to NaF in SEI on the surface of the electrolyte membrane, which is electrochemically favorable and chemically stable. The 19F NMR spectra confirm the existence of the NaF signal at −226 ppm. The 19F (19,40) resonances at −182.8 and −185.3 ppm are from the −CF groups of the −HFP segment, and the rest of the resonances are attributed to regional irregular structures (−119.6 and −111.6 ppm) and the crystalline domain (−93.0 ppm) of −CF2 groups, as well as −CF3 groups (−76.5 ppm). (41,42) The generated NaF allows Na fast ionic conduction of ions for uniform Na striping/plating during cycling, promoting the electrochemical stability performance of battery cycles. (40)

4. Conclusions
ARTICLE SECTIONS
In summary, we have designed and prepared a highly porous, single-ion conducting polymer electrolyte composed of a novel sodium salt (NaPTAB) and PVDF–HFP using inexpensive and environmentally friendly raw materials and easy synthetic steps. NaPTAB and NaPTAB-SM have a high thermal decomposition temperature of about 345 °C. The GPE (PC swelled NaPTAB-SM) exhibits significantly ionic conductivity over a wide temperature range of 30–100 °C, a favorable electrochemical window, and a high sodium-ion transference number. Excellent performance of the NVP/Na batteries using NaPTAB-SGPE was reported, such as good charge/discharge ability and stable cycle performance at high temperatures. We reveal that the NaF component in SEI is responsible for the excellent stable cycling performance of the sodium-ion battery for the NaPTAB-SGPE polymer electrolyte membrane compared to the commercial NaClO4 liquid electrolyte. In summary, this gel polymer electrolyte has superior performances and can make it very a promising candidate for sodium batteries, especially at high temperatures.
【Article link】
https://doi.org/10.1021/acsaem.0c01756