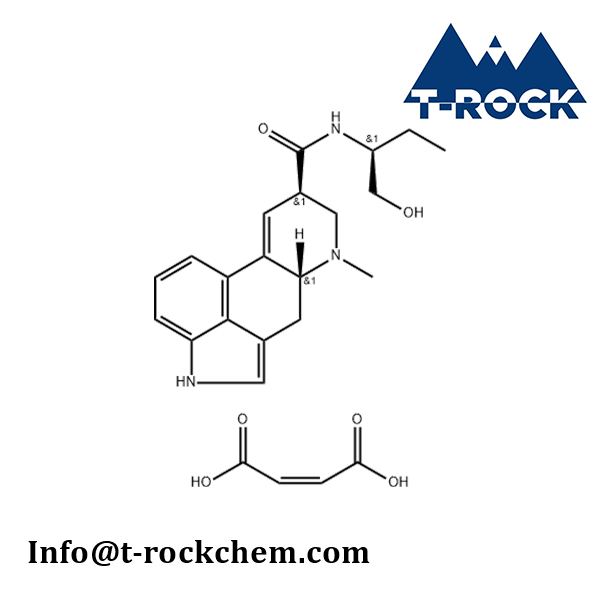
Methylergometrine Maleate
CAS: 57432-61-8
Category: Pharmaceutical IntermediatesRelated products
-
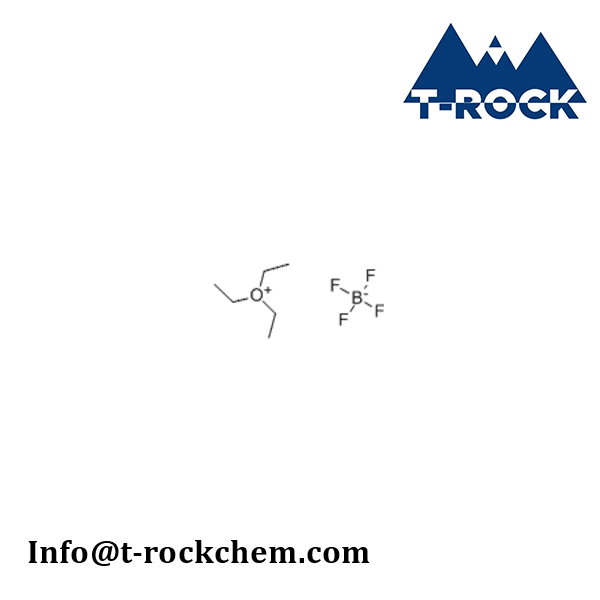
Triethyloxonium tetrafluoroborate
CAS: 368-39-8
-
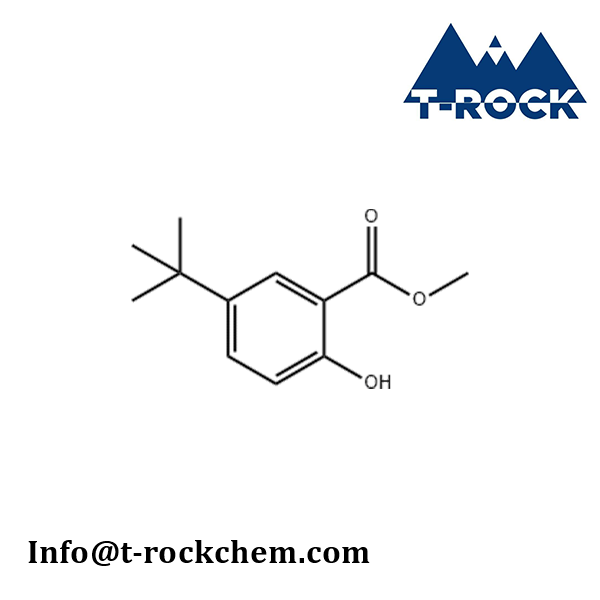
METHYL 5-TERT-BUTYL-2-HYDROXYBENZOATE
CAS: 52888-72-9
-
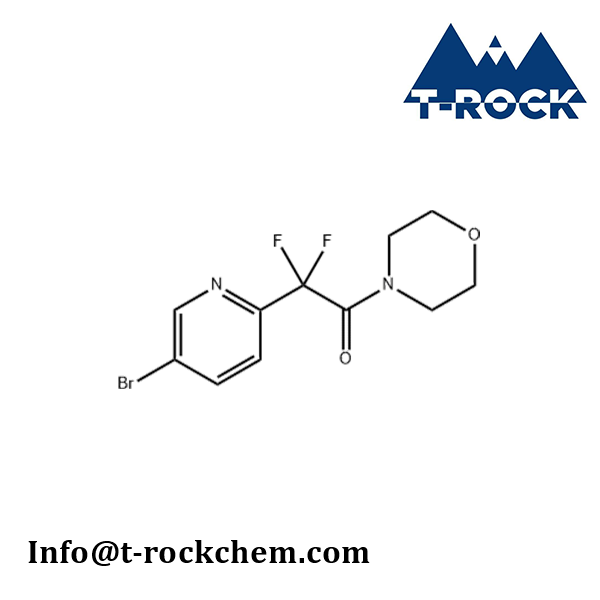
2-(5-bromopyridin-2-yl)-2,2-difluoro-1-morpholinoethan-1-one
CAS: 1809323-20-3
-
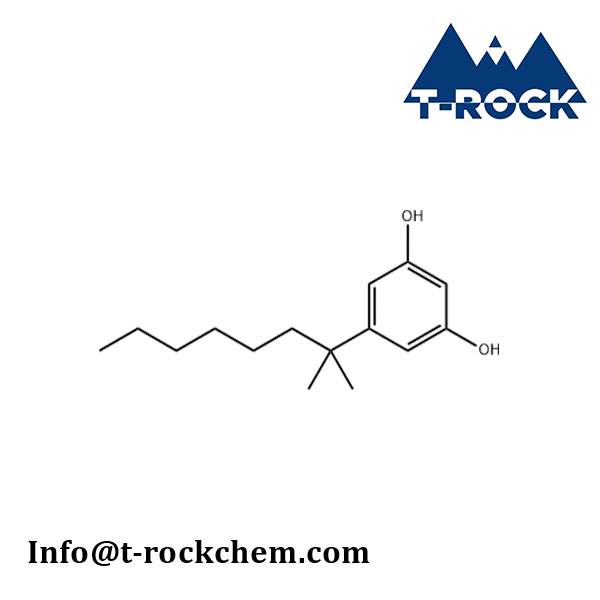
5-(1,1-Dimethylheptyl)resorcinol
CAS: 56469-10-4
