Liao Shen More by Liao Shen, Yadan Zhang, Jinlong Zhang, Tao Wang, Haiyan Li, Yongan Wang*, and Dongqin Quan* Cite this: ACS Appl. Mater. Interfaces 2019, 11, 23, 20642–20648 Publication Date:May 22, 2019
Abstract
Transarterial chemoembolization (TACE) is a promising treatment for patients suffering from unresectable liver malignancy. A coarse emulsion of doxorubicin solution and iodized oil is widely used in clinical practice. However, this coarse emulsion lacks sufficient physical stability and can split into water and oil very quickly. Furthermore, most chemotherapeutics are quickly released into systematic circulation, causing serious adverse effects. In this study, we aimed to prepare reversed lipid-based nanoparticles (RLBNs) dispersed in iodized oil as nanocarriers for the delivery of hydrophilic chemotherapeutics. Unlike a simple mixture of drug solution and oil, RLBN is a homogenous system and possesses a hydrophobic nanostructure that has high dispersibility in oils. Hydrophilic chemotherapeutics were entrapped in the polar core juxtaposed by highly biocompatible lipid materials, such as egg phospholipids. A sustained drug-release profile was observed in both in vitro and in vivo pharmacokinetics studies. The results of computed tomography showed that RLBN–doxorubicin–iodized oil could remain in the tumor region for more than 14 days and that the growth of tumors was effectively suppressed. Thus, the current results suggest that RLBN is a promising drug delivery system and is compatible with TACE treatment.
1. Introduction
Hepatocellular carcinoma is one of the most common cancers worldwide. (1) Hepatectomy is still the first choice of treatment. However, approximately 80% of patients who suffer from primary or secondary liver malignancy cannot undergo surgery. (2,3) Systematic chemotherapy has a limited antitumor effect in clinical practice; besides, serious adverse effects, such as cardiotoxicity and marrow toxicity, might be lethal to patients. (4,5) Therefore, the treatment for liver cancer remains challenging.
Transarterial chemoembolization (TACE) is a promising method for the treatment of hepatocellular carcinoma. (6,7) One of the purposes of TACE is to increase the efficiency of drug delivery to the tumor region, while reducing drug distribution to normal tissues, thus minimizing adverse effects. Iodized oil is commonly used in hepatic artery embolization because it can be metabolized in normal liver tissues, but is nondegradable in the tumor. (8) Hence, iodized oil is also an ideal radiocontrast agent that could be monitored by X-ray. A simple mixture of drug solution and iodized oil is widely used in TACE. (6) Although the mixture possesses relatively higher antitumor effects than that of hepatic arterial infusion of drug solution, it is prone to rapid release into the systemic circulation. The poor physical stability of the mixture of drug and iodized oil may also lead to serious inconvenience in clinical practice. (9) A drug-eluting bead is reported as a novel drug carrier that has drawn considerable attention in recent years. (2,10,11) Although its therapeutic effect has been proved in patients, the nondegradable materials may pose potential hazards in the long run. Furthermore, high production cost leads to a high price of drug-eluting beads.
In our previous study, we prepared reversed lipid-based nanoparticles (RLBNs) loaded with a typical hydrophilic therapeutic agent, doxorubicin (DOX) chloride. (12) Biocompatible amphipathic lipid materials (egg phosphatidylcholine; EPC) were used to juxtapose a layer around the polar core as a drug reservoir. The hydrophilic therapeutic agent was entrapped in the hydrophobic nanostructure and thus the RLBN could be dispersed in liquid oils as a homogeneous system. A sustained drug-release profile and an enhanced antitumor effect were observed in animals (nude mice bearing solid tumor) intratumorally injected with RLBN. In the present study, we aimed to develop RLBN–DOX–IO (reversed lipid-based nanoparticle–doxorubicin–iodized oil) using iodized oil as the dispersion medium and DOX as a model drug for hepatic chemoembolization treatment. Unlike a simple mixture of DOX and iodized oil, RLBN–DOX–IO featured a more stable homogenous phase that would not split into oil and water for a long time. Thus, RLBN–DOX–IO is easier to use in TACE surgery and the antitumor activity of chemotherapeutics could also be enhanced by longer drug retention time in the tumor region. The size distribution of RLBN–DOX–IO was measured by dynamic light scattering (DLS). Wistar rats bearing liver tumors were used as the animal model to perform the in vivo evaluation. RLBN–DOX–IO was administrated via a catheter placed in the gastroduodenal artery and the tumor sizes and formulation retention in the tumor region were monitored by computed tomography (CT) that lasted 14 days.
2. Materials and Methods
2.1. Materials
EPC and dipalmitoyl phosphatidylglycerol (DPPG)-Na were purchased from Lipoid GmbH (Ludwigshafen, Germany); iodized oil was purchased from Hunan ER-KANG Pharmaceutical Co., Ltd. (Changsha, Hunan Province, China). DOX was obtained from Zhejiang Hisun Pharmaceutical Co., Ltd. (Taizhou, Zhejiang Province, China). Dulbecco’s modified Eagle’s medium and fetal bovine serum were obtained from Gibco Co. (Paisley, U.K.). The antibiotics (100 U/mL penicillin and 100 U/mL streptomycin) and nonessential amino acids were purchased from Sigma-Aldrich Co. Lipiodol was purchased from Guerbet (France). All other chemicals were of analytical reagent grade or better.
2.2. Preparation and Characterization of RLBN–DOX–IO
As the first step, lipid dispersion without the drug was prepared by the film-dispersion and hydration–sonication process as described previously. (12) Briefly, 8 mL of chloroform was used to dissolve the phospholipids DPPG-Na and EPC at a ratio of 8:92 (w/w). Double distilled water (10 mL) was added to obtain the phospholipid suspension after the removal of organic solvent by evaporation. A probe-type sonicator (200 W for 3 min on ice bath) was used to form empty single unilamellar vesicles (SUVs). The SUV solution was mixed with DOX solution (6 mg/mL) and stirred for 10 min. The moisture from the sample was then removed by lyophilization. The freeze-dried products were dispersed in iodized oil by stirring at 25 °C. Finally, a clear RLBN–DOX–IO dispersion was obtained. The size distribution of RLBN–DOX–IO was measured by dynamic light scattering (DLS); iodized oil was used as the solvent and the relative parameters were reset for the measurement (viscosity = 25 cP, refractive index = 1.5104, temperature = 37 °C). All procedures were performed in a dark environment to avoid the degradation of DOX.
2.3. In Vitro Drug Release
Phosphate-buffered saline (PBS, pH = 7.4) was used as the release medium to study the in vitro release pattern of RLBN–DOX–IO, a DOX–IO coarse emulsion, and a simple mixture of iodized oil and DOX. Specifically, 2 mL of the RLBN–DOX–IO solution was added to 30 mL of PBS and stirred at 100 rpm in a 37 °C water bath. Samples were withdrawn and replaced with an equal volume of fresh PBS at 1, 2, 5, 8, 12, 24, 48, 72, 96, and 120 h. For the simple mixture and coarse emulsion groups, samples were withdrawn and replaced with equal volumes of fresh PBS at 0.5, 1, 3, 5, 15, 30, 60, 90, and 120 min. The concentration of DOX in the medium was determined immediately by high-performance liquid chromatography (column: Zorbax SB-C, 250 mm × 4.6 μm, 5 μm; mobile phase: water, acetonitrile, and phosphoric acid (52:48:0.68) with 1.44 g/L sodium lauryl sulfate, pH = 4.0 ± 0.1; detection wavelength = 254 nm). Cumulative release of DOX was calculated as follows

Where V0 is the initial volume, Vs is the sampling volume, C0 and Cn are the drug concentrations, n is the sampling time, and M is the total mass of drug in the formulation. All assays were performed in the absence of light.
2.4. In Vivo Evaluation in Tumor-Bearing Rats
Wistar rats (150–180 g, specific pathogen-free) were used as experimental animals in this study. To establish the liver cancer model, 0.1 mL of C6 cell suspension (2.0 × 107 cells in 100 μL PBS) was injected into the left lateral lobe of the liver of each animal. The tumor size reached ∼100 mm3 after 10–14 days. All animals were randomly divided into three groups, viz., RLBN–DOX–IO, DOX–IO, and DOX–SOL groups. A 0.1 mL aliquot of RLBN–DOX–IO (4 mg/mL DOX), DOX–IO (DOX solution mixed with iodized oil, containing 4 mg/mL DOX), or saline was administered to animals under anesthesia from each group, via a catheter placed in the gastroduodenal artery. The day on which the animals received the drug administration was set as day 0.
2.4.1. Pharmacokinetics Study
For blood sampling, catheters were inserted in the jugular vein of each rat 2 days before drug administration. At predetermined intervals, 0.2 mL of blood was collected from each animal via the catheter. The concentration of DOX in each sample was quantified by liquid chromatography with tandem mass spectrometry (LC–MS/MS) after pretreatment.
2.4.2. Concentration of DOX in Tumors
On days 1, 3, and 10, three rats in each group were euthanized and the tumors in the liver were harvested immediately. The concentration of DOX in each tumor was analyzed by LC–MS/MS after pretreating the samples.
2.4.3. Computed Tomography
On days 0, 7, and 14, six rats from each group were scanned by a computed tomography device (CT; Angio-CT System, Siemens) to record the tumor size. All animals were under mild anesthesia to obtain stable and accurate images. The tumor size was estimated by its largest (L) and smallest (S) diameters as follows

2.5. Histological Analysis
All rats were euthanized on day 14. Hearts, normal liver tissues, and tumors in the liver were individually harvested. The samples were immediately immersed in 10% formalin and embedded in paraffin. The embedded specimens were sectioned and stained with hematoxylin and eosin (H&E). Apoptotic cells in tumor tissues were labeled using an In Situ Cell Death Detection Kit (TUNEL assay; Roche, Berlin, Germany), and the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). All of the processes were performed according to standard operating procedures and the slides were examined using an inverted fluorescence microscope (NIKON ECLIPSE TI-SR; Nikon, Tokyo, Japan).
2.6. Statistical Analyses
All data are presented as mean ± standard deviation (SD). Either the Student’s t-test or a two-way analysis of variance (ANOVA) was used to evaluate the data. The results with a p-value <0.05 were considered statistically significant.
3. Results
3.1. Preparation and Characterization of RLBN–DOX–IO
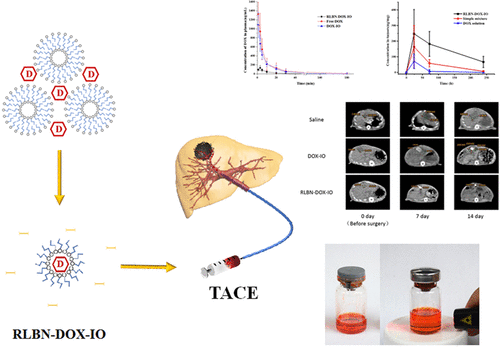
As shown in Figure 1a, RLBN–DOX–IO is a clear and homogeneous red suspension. No sediment was observed in the RLBN–DOX–IO solution after 2 weeks in the dark environment under 25 °C. A simple mixture of DOX powder and iodized oil is heterogeneous and physically unstable, limiting its use under any practical conditions. The coarse emulsion of DOX solution and iodized oil, which is commonly used in clinical TACE, quickly splits into two layers (within 1 min) under 25 °C. Figure 1b shows the Tyndall effect of RLBN–DOX–IO, indicating the colloidal state of the system. The size distribution is shown in Figure 1c. The average size of RLBN–DOX in iodized oil was approximately 100 nm and the polymer disperse index was 0.179.

3.2. In Vitro Drug Release
Cumulative drug-release kinetic curves are shown in Figure 1d. The release profile of RLBN–DOX–IO can be described in two phases, namely a fast-release phase and a sustained-release phase. In the fast-release phase, approximately 18% of the drug is released in 10 h, while in the sustained-release phase, an additional 10% of the drug is slowly released over a 5 day period. The simple mixture and coarse emulsion of DOX and iodized oil showed different drug-release patterns. For the simple mixture, cumulative drug release increased gradually from 0 h as shown in Figure 1e (black curve). Though sustained drug release was observed with the simple mixture, DOX was released from the iodized oil phase much faster than from RLBN–DOX–IO, requiring only 2 h to release 94.08 ± 2.51% of the drug. Drug release from the coarse emulsion of DOX and iodized oil occurred rapidly, where almost all of the DOX distributed into the water phase within 15 min.
3.3. In Vivo Evaluation in Tumor-Bearing Rats
3.3.1. Pharmacokinetics Study
The changes of DOX concentration in plasma of each group are shown in Figure 2. In the DOX–SOL and DOX–IO groups, the DOX concentration peaked immediately after artery perfusion (1324.78 ± 263.05 ng/mL in the DOX–SOL group and 1087.16 ± 277.95 ng/mL in the DOX–IO group, Table 1), and then sharply decreased and returned to almost the baseline value at 30 min (61.14 ± 52.95 ng/mL in the DOX–SOL group and 49.72 ± 47.12 ng/mL in the DOX–IO group, Table 1). The pattern of change in the DOX concentration in these two groups matched the general characters of intravenous injection of DOX solution. In the RLBN–DOX–IO group, the DOX concentration reached the peak concentration (132.95 ± 52.82 ng/mL) at 3 min, which was significantly lower than that in the other two groups (p < 0.001), and then slowly decreased. From 10 to 180 min, although the mean DOX concentration in the RLBN–DOX–IO group was lower than that in the DOX–SOL and DOX–IO groups, there was no significant difference among the three groups. The AUC0–∞ of the three groups was 130 08.84 ± 4098.88 (DOX–SOL), 10 191.17 ± 2505.43 (DOX–IO), and 1577.88 ± 672.54 min·ng/mL (RLBN–DOX–IO), indicating limited drug release from RLBN–DOX–IO into peripheral blood circulation.

Table 1. Pharmacokinetics Resultsa,b
| parameter | unit | DOX–SOL | DOX–IO | RLBN–DOX–IO |
|---|---|---|---|---|
| AUC0–t | min·ng/mL | 12 604.41 ± 3868.14 | 10 146.23 ± 2440.15# | 1529.06 ± 649.77***,@@@ |
| AUC0–∞ | min·ng/mL | 13 008.84 ± 4098.89 | 10 191.17 ± 2505.43# | 1577.89 ± 672.54***,@@@ |
| t1/2 | min | 26.85 ± 8.72 | 24.47 ± 2.39 | 20.98 ± 9.60 |
| Cmax | ng/mL | 1367.28 ± 298.50 | 1087.16 ± 277.95 | 135.93 ± 50.10 |
| Tmax | min | 1.40 ± 0.89 | 1.00 ± 0.00 | 2.60 ± 0.89 |
| MRT0–t | min | 20.65 ± 14.41 | 17.92 ± 3.08 | 12.57 ± 2.59 |
a
Data are presented as mean ± SD, n = 5.
b
RLBN–DOX–IO: reversed lipid-based nanoparticles loaded with DOX and dispersed in iodized oil; DOX–IO: a coarse emulsion of DOX and iodized oil; AUC: area under the curve; MRT: mean residence time. DOX–IO vs DOX–SOL, #p < 0.05; RLBN–DOX–IO vs DOX–SOL, ***p < 0.001; RLBN–DOX–IO vs DOX–IO, @@@p < 0.001.
3.3.2. DOX Concentration in Tumor
The intratumoral DOX concentration-kinetic curve is shown in Figure 3. In the DOX–SOL group, the DOX concentration in the tumor was 5.20 ± 5.94 ng/mg at 24 h, whereas DOX was undetectable on days 3 and 10. In the DOX–IO group, the intratumoral DOX concentration was 35.12 ± 21.77 and 12.64 ± 4.93 ng/mg on days 1 and 3, respectively; DOX was undetectable on day 10. Compared with that in the DOX–SOL and DOX–IO groups, the DOX concentration in the tumor was high in the RLBN–DOX–IO group on days 1, 3, and 10; the DOX concentration was 230.24 ± 105.93, 92.98 ± 56.97, and 36.44 ± 27.96 ng/mg, respectively. A multiple comparison test by two-way ANOVA showed significant differences in the DOX concentration on days 1 and 3 in RLBN–DOX–IO versus DOX–SOL and RLBN–DOX–IO versus DOX–IO, respectively. No statistical difference was observed between the DOX–SOL and DOX–IO groups.

3.3.3. Computed Tomography
Changes in tumor size in each group were determined by CT (Figure 4). Without treatment, the tumors grew from 124.24 ± 46.80 to 789.51 ± 345.98 mm3, whereas the average volume increased by about 6-fold after 2 weeks. In the DOX–IO group, the tumor size increased by approximately 3-fold at the end of the experiment. RLBN–DOX–IO effectively inhibited tumor growth, as evidenced by the mean tumor volume on days 0 and 14, which was 137.47 ± 75.38 and 160.65 ± 96.39 mm3, respectively. DOX–IO suppressed tumor growth within 7 days; however, the CT results showed obvious individual differences among experimental subjects (789.51 ± 345.98 mm3, day 14). Thus, rapid tumor regrowth was observed in some rats after 7 days. In contrast, the therapeutic effect of RLBN–DOX–IO was statistically significant compared with that of DOX–IO and the tumor inhibition effect was more stable throughout the 14 day observation period.

3.4. Histological Analysis
3.4.1. H&E- and TUNEL-Stained Histological Sections of Tumors
H&E-stained histological sections of tumors treated with saline, DOX–SOL, and RLBN–DOX–IO on day 14 are shown in Figure 5a. No significant necrosis was observed in the saline group and several small regions of necrosis were observed in the DOX–IO group. In the RLBN–DOX–IO group, a relatively larger necrosis area was observed, suggesting low activity of tumor tissues. Blood vessels were clearly observed in the saline and DOX–IO groups, which are indicated by yellow arrows in the figure.

Apoptotic cells were stained by the TUNEL assay (fluorescing green) and the nuclei in live cells were stained by DAPI (fluorescing blue). As shown in Figure 5b, only a few apoptotic tumor cells were found in the saline group, suggesting that the tumor cells were highly viable. Apoptotic tissues were clearly observed in the DOX–SOL group, whereas a large proportion of viable tumor cells fluorescing bright blue were also observed, indicating the potential reoccurrence of neoplasm. In the RLBN–DOX–IO group, most areas were stained green and only weak blue fluorescence was observed, indicating that tumor cell viability was significantly suppressed. Red fluorescence in the RLBN–DOX–IO group (Figure 5c) showed residual DOX in the tumor region, suggesting prolonged retention of the formulation.
3.4.2. Histological Analysis of the Heart and Liver
The H&E-stained histological sections of the heart and liver are shown in Figure 5d (left: heart; right: liver). No significant pathological alteration was observed in the heart sections on days 1 and 14, suggesting that neither DOX–IO nor RLBN–DOX–IO induced severe heart failure by transarterial chemoembolization. In the liver sections, several lipid vacuoles were observed (indicated by yellow arrows) in the DOX–IO and RLBN–DOX–IO groups on day 1, which may be caused by residual iodized oil; this alteration diminished on day 14. Nondifferential light inflammatory infiltrations were found in the DOX–IO and RLBN–DOX–IO groups on day 14 (indicated by red arrows), demonstrating that RLBN–DOX–IO did not aggravate liver damage compared with that by conventional TACE.
4. Discussion
Local chemotherapy has been widely applied for patients with hepatocellular carcinoma who are not suitable for surgical excision. Recently, advanced imaging technologies such as digital subtraction angiography (DSA) and computed tomography have allowed surgeons to locate the main blood vessel feeding a tumor in real time while implementing chemoembolization. The most common embolic agents are drug-eluting beads and iodized oil. The drug-eluting beads have drawn considerable attention owing to their relatively superior and long-term anticancer effect. However, it is also important not to ignore the risks of implanting such nonbiodegradable materials in the blood vessels that may be washed into the periphery blood circulation system. Iodized oil is one of the ideal embolic agents because it cannot be metabolized in the tumor, but is degradable in normal liver tissues. Moreover, numerous anticancer chemotherapeutics are not soluble in oily solvent, and thus, a coarse emulsion of drug solution and iodized oil is used in clinical practice. Apparently, owing to the lack of surfactants and proper pretreatment, the coarse emulsion is physically unstable and pharmaceutically uncontrollable. Moreover, hydrophilic drugs remain in the water phase and are quickly released into the blood, reducing their therapeutic effect. In the present study, we prepared a novel “oil-soluble” lipid-based nanoparticle that could load and suspend hydrophilic chemotherapeutics homogeneously in iodized oil for TACE. We named the drug delivery system RLBN. The RLBNs possess the unique “reversed” structure shown in Scheme 1. Phospholipids were used to juxtapose a monolayer, while the hydrophilic groups of the phospholipids formed a polar core that entrapped the chemotherapeutic agent. Furthermore, the fatty acid chains of the phospholipids formed the outer shell of the nanoparticle that made it “soluble” in oily solvent. We used DOX as the model drug because of its high hydrophilic character and the solid anticancer activity on the malignant tumor. The natural phospholipid EPC and the synthetic DPPG were used as the membrane materials to prepare negatively charged lipid vesicles as the first step in the preparation of RLBN. In the next step, the DOX solution was mixed with lipid vesicles. The DOX molecules are positively charged in water and could form supramolecular assemblies with negatively charged lipid vesicles by a strong electrostatic interaction. The moisture content in the solution was removed by freeze-drying, leaving DOX molecules in the space between lipid vesicles. Iodized oil was then added to suspend the freeze-dried cake. During this process, the phospholipid monolayer rearranged to entrap DOX molecules in the polar core, then dispersed homogeneously in the oil phase and formed nanosized RLBNs.

Unlike in the coarse emulsion, the drug molecules in RLBN stay in the oil phase and are entrapped in oily nanoparticles. Therefore, the RLBN drug delivery system is a homogeneous system and could be physically stable, features that are quite important in TACE clinical practice. During operation, a surgeon may need adequate time to locate the main blood vessel feeding a tumor under DSA. Making an iodized coarse emulsion just before the injection is certainly time-consuming and sophisticated. On the other hand, neither the simple mixture nor the coarse emulsion preparations are homogenous systems, leading to large uncertainties in dosing and poor pharmaceutical quality control. RLBN could offer an accurate dose and be easy to use during operation, making precise adjustments to chemotherapeutic dose possible and lowering the risk of surgery failure.
Another advantage of RLBN–DOX–IO is the sustained drug-release pattern, where the drug-release process may be dramatically slowed by the oil–water interface barrier leading to a prolonged local antitumor activity. Compared with RLBN–DOX–IO, the coarse emulsion has an apparent initial burst phase of drug release in vitro and a peak concentration that is reached within 15 min; the simple mixture releases more than 90% of the drug after 2 h. Theoretically, this could lead to a situation where most of the chemotherapeutic agents circulate in the blood, rather than reaching the tumor region, causing severe side effects and lowering the antitumor effect.
To evaluate the antitumor effect of RLBN–DOX–IO in vivo, we generated a tumor-bearing animal model by injecting a C6 cell suspension into the livers of Wistar rats. As iodized oil is also an ideal contrast agent, we could use CT to monitor formulation retention and tumor size by a noninvasive method. In the saline group, the tumors increased by 6-fold in 14 days, suggesting high tumor activity in the animal model. Coarse emulsions are widely used in clinical practice and the coarse DOX–IO emulsion also showed inhibitory effects on tumors in our animal model. However, the pharmacokinetic study results show that although the DOX–IO emulsion was administered by transarterial perfusion, the drugs still spread very quickly to the peripheral circulatory system, leading to locally low DOX concentrations in tumors and greatly weakening the antitumor effect. Furthermore, transarterial embolization may not entirely block all of the blood vessels supplying tumors with only one perfusion; thus, tumors could induce collateral blood vessels and rebuild the nutrition supply. This phenomenon is quite common in clinical practice and also one important reason for the recurrence of tumor. Residual DOX determination in tumor showed that RLBN–DOX–IO could maintain a high long-term intratumoral chemotherapeutic concentration, thus imposing a strong effect on the tumor through the combined cytotoxic effect and blocking of blood supply. Pharmacokinetic studies also showed that less DOX was released into the peripheral blood circulation from the RLBN–DOX–IO formulation, which could reduce drug distribution to normal tissue and reduce side effects such as cardiotoxicity.
Histological analysis showed a stronger antitumor activity of RLBN–DOX–IO than that of conventional TACE by DOX–IO. Moreover, a red fluorescence scan of tumor sections also presented more residual DOX in the RLBN–DOX–IO group on the last day of the experiment, proving the sustained drug-release characteristic of RLBN. No damage to the heart was observed in any of the groups, which may be explained by low-drug dosage in a single administration. Lipid vacuoles were observed on day 1 and disappeared before day 14. This is because the normal hepatic tissues could metabolize iodized oil, owing to the existence of Kupffer cells. A light inflammatory response was observed in the RLBN–DOX–IO group, but there was no significant difference compared with the DOX–IO group, suggesting that RLBN–DOX–IO did not enhance the liver damage compared with conventional TACE.
5. Conclusions
In this study, we prepared reversed lipid-based nanoparticles to load hydrophilic chemotherapeutic DOX chloride and used iodized oil as the dispersion medium. Compared with conventional TACE, which uses a simple mixture of DOX water solution and iodized oil, RLBN–DOX–IO is not only user-friendly but also has a significantly sustained drug-release profile with lower peripheral drug distribution. In vivo anticancer evaluation in tumor-bearing rats showed enhanced tumor growth inhibition by RLBN–DOX–IO. Results showed that the RLBN drug delivery system could overcome some drawbacks in conventional TACE and may be suitable for clinical use. Although further studies are required, the present study results show that RLBNs have potential as a local chemotherapy delivery system for TACE.
【Article link】